To: Administrative File: CAG #00410N
Outpatient Intravenous Insulin Therapy Regimen
From: Louis Jacques, M.D.
Director, Coverage and Analysis Group
Tamara Syrek Jensen, J.D.
Deputy Director, Coverage and Analysis Group
Marcel E. Salive, M.D., M.P.H.
Director, Division of Medical and Surgical Services
Sandra Jones, R.N., M.S.
Analyst
Elizabeth Koller, M.D., F.A.C.E.
Medical Officer
Subject: Coverage Decision Memorandum for Outpatient Intravenous Insulin Therapy Regimen
Date: December 23, 2009
I. Decision
- The Centers for Medicare and Medicaid Services (CMS) has determined the following.
The evidence does not support a conclusion that outpatient intravenous insulin therapy improves health outcomes in Medicare beneficiaries. Therefore, CMS has determined that outpatient intravenous insulin therapy is not reasonable and necessary for any indication under section 1862(a)(1)(A) of the Social Security Act. Services comprising an Outpatient Intravenous Insulin Therapy regimen are nationally noncovered under Medicare when furnished pursuant to an outpatient intravenous insulin therapy regimen.
- Outpatient Intravenous Insulin Therapy (OIVIT) consists of an outpatient regimen of pulsatile or continuous intravenous infusion of insulin via any means, guided by the results of:
- measurement of respiratory quotient; and/or
- measurement of urine urea nitrogen (UUN); and/or
- measurement of arterial, venous or capillary glucose; and/or
- measurement of potassium concentration;
performed in scheduled recurring intermittent episodes.
This regimen is also sometimes termed Cellular Activation Therapy (CAT), Chronic Intermittent Intravenous Insulin Therapy (CIIIT), Hepatic Activation Therapy (HAT), Intercellular Activation Therapy (iCAT), Metabolic Activation Therapy® (MAT®), Pulsatile Intravenous Insulin Treatment (PIVIT), Pulse Insulin Therapy (PIT) and Pulsatile Therapy (PT).
II. Background
Terminology
The term outpatient intravenous (IV) insulin therapy (OIVIT) refers to an outpatient regimen that integrates pulsatile or continuous intravenous infusion of insulin via any means, guided by the results of:
- measurement of respiratory quotient; and/or
- measurement of urine urea nitrogen (UUN); and/or
- measurement of arterial, venous or capillary glucose; and/or
- measurement of potassium concentration;
performed in scheduled recurring intermittent episodes.
The term diabetes refers to diabetes mellitus (DM) unless we describe a more specific use. We specify Type 1 or Type 2 DM when the discussion refers to one type rather than to both. The terms hypo- and hyperkalemia refer respectively to abnormally low or high levels of potassium (from the Latin kallium) in the blood. The terms hypo- and hyperglycemia refer respectively to abnormally low or high levels of glucose (from the Greek glykys) in the blood. The term endogenous is an adjective referring to internal bodily processes or the products of internal bodily processes. Glycogen is a large molecule consisting largely of multiple glucose molecules linked together, and serves as a means to store energy. The term hepatic is an adjective that refers to the liver.
Proteins are comprised of amino acids, many of which are derived from dietary sources. A peptide may be thought of as a small fragment consisting generally of fewer amino acids than a protein. Insulin is an example of a peptide hormone.
A cell may store substances in tiny packets called vesicles, or substances may be dispersed more generally in the intracellular liquid environment (cytosol). Cells release substances through a variety of mechanisms. Small molecules may traverse the cell membrane via specific gates or channels. Cells may also release substances through a process called exocytosis, by which a vesicle will merge with the cell’s membrane and discharge its contents directly into the surrounding environment.
Human cells display electrochemical activity to various degrees, depending on their biologic function. The electrical properties of cells are determined by the relative concentrations of electrically charged atoms (ions) inside and outside of the cell. Sodium, potassium, calcium and chloride are examples of ions that play a significant role. When various stimuli cause these ions to move into or out of the cell in a coordinated manner the cell becomes momentarily "depolarized." Energy is required to maintain this electrical balance. Adenosine triphosphate (ATP) is a nucleotide molecule which stores cellular energy in its bonds to its three phosphates; adenosine monophosphate (AMP) and adenosine diphosphate (ADP) store lower amounts of cellular energy with their one and two phosphate units respectively. A kinase is a type of enzyme. Additional terms are defined below as needed.
Scope of this decision
We recognize that various individual components of OIVIT, e.g., insulin, insulin infusion, pump use, metabolic cart measurements, and laboratory testing, may have medical uses in conventional treatment regimens for diabetes and other conditions. We describe many of the more conventional uses of these components in the background sections that follow. In this decision, we are not making (a) coverage determination(s) regarding those other various uses. Coverage for such other uses may be determined by other local or national Medicare determinations and will not be considered here.
We remind the reader that Medicare differentiates the clinical laboratory diagnostic glucose testing performed by medical professionals from the home self-monitoring of glucose that is customarily performed by patients. The test equipment and supplies for home self-monitoring are coverable under the durable medical equipment (DME) benefit and are not payable as diagnostic tests for Medicare purposes. As such, they are not included in the scope of this review.
Commercially available insulin preparations
Various preparations of insulin have FDA approved labeling to improve glycemic control in adults and children with diabetes mellitus. The commercially available insulin products are marketed by many manufacturers. The original animal-derived insulins (beef, pork) have been largely supplanted by recombinant (genetic technology) human insulin or recombinant insulin analogues, (Brogden 1987, Chance 1993, Heller 2007, Hoome 1982, Ladisch 1992) The pharmacokinetic and pharmacodynamic activity profiles of the various insulin products depend on changes in the primary molecular structure of the insulin (native insulin versus insulin analogue), the addition of modifying components such as zinc and protamine, and the route of administration (subcutaneous versus intravenous). (Bruni 1973, Hagedorn 1936, Meneghini 2008, Scott 1935) There are unlabelled uses of IV insulin unrelated to diabetes mellitus, such as the rapid correction of emergent hyperkalemia in the setting of cardiac toxicity. (Allon 1990, Birmbaum in Olson 2004, Hollander-Rodriguez 2006, Kocoglu 2002)
Endogenous insulin
The pancreas is a gland that has both exocrine (digestive enzymes) and endocrine (digestive hormones) functions. The specialized areas producing hormones are islets (of Langerhans) and are scattered throughout the pancreas. The various islets contain "alpha", "beta", "delta", and "epsilon" cells, which secrete the hormones "glucagon", "insulin", "somatostatin", and "ghrelin" respectively. (Andralojc 2008, Dezaki 2006, Docherty 2001, Gromada 2007) Insulin is produced as proinsulin, which is cleaved in the beta cells to produce insulin and C-peptide. C-peptide has no definitively established metabolic effect, but is measurable as a marker for insulin production by the pancreas and to differentiate endogenously produced insulin from administered insulin preparations in blood samples. (Brandenburg 2008, Hills 2008, Norquist 2008)
Insulin and its counter-regulatory hormone, glucagon, are released into the portal vein, through which they enter the liver. The portal venous system is anatomically unique in that venous blood from the small intestine is directed to the liver rather than returning directly through the systemic (general) venous system to the heart. Thus insulin, glucagon, digested nutrients and drugs may be found in higher concentrations in the portal vein than in the systemic venous circulation, and may act upon or be acted upon by the liver before entering the systemic venous circulation. In contrast, digestive enzymes are released into the pancreatic duct and subsequently enter the common bile duct, from which they are delivered into the duodenal part of the small intestine through a natural opening called the ampulla of Vater.
As noted above, insulin has uses beyond diabetes. However, because it is illustrative of insulin’s biochemical actions and clinical effects, we have included a detailed discussion of diabetes and insulin below. We recognize that this discussion may be complex for the lay reader, but the underlying topic is itself complex, and we believe that oversimplification will lead to misinterpretation and misunderstanding.
Diabetes
Diabetes encompasses a spectrum of metabolic disorders. Classical Type 1 DM (previously termed Juvenile Diabetes) is an autoimmune disorder in which there is destruction of the pancreatic islet cells that produce insulin, and sometimes also the islet cells that produce counter-regulatory hormones that mitigate hypoglycemia. Because they lack endogenous insulin, Type 1 diabetic patients require insulin replacement in multiple doses throughout the day to prevent ketoacidosis. This contrasts with Type 2 DM (previously termed Adult-Onset Diabetes), in which insulin is still produced, but is secreted in insufficient quantities to meet insulin requirements because of impaired insulin action (resistance). These patients do not require daily insulin to avoid ketoacidosis, but may benefit from insulin supplementation to correct nocturnal hyperglycemia or post-prandial (after eating) hyperglycemia. Patients with mixed disorders may require therapeutic intervention with modalities and regimens from both Type 1 and Type 2 diabetes.
Physiology of insulin secretion
In the following section, we describe the physiology of insulin secretion. We believe this is important because some proponents of OIVIT cite the importance of mimicking the endogenous release of insulin in support of their claims.
Glucose utilization by the body is mediated by both insulin and non-insulin mediated mechanisms (Figure 1). (Baron 1987, 1988, Saltiel, Pessin 2007) The beta cells in the pancreatic islets secrete insulin into the hepatic portal vein (splenic-mesenteric confluence), which drains the mesenteric (largely digestive) organs and supplies the liver. (Bergman 2000, Moore 2003) Insulin levels in the portal vein are substantially higher than in the hepatic vein, which drains the liver, and in other peripheral veins. Exposure to high insulin levels results in suppression of hepatic gluconeogenesis (glucose formation) and an increase in hepatic glycogenesis (glycogen formation). Insulin exposure in the peripheral circulation causes glycogenesis in muscle tissue and triglyceride disposition in adipose (fatty) tissue. (De Meyts 2000)
Figure 1: Pictorial of Glucose Utilization and the Role of Insulin
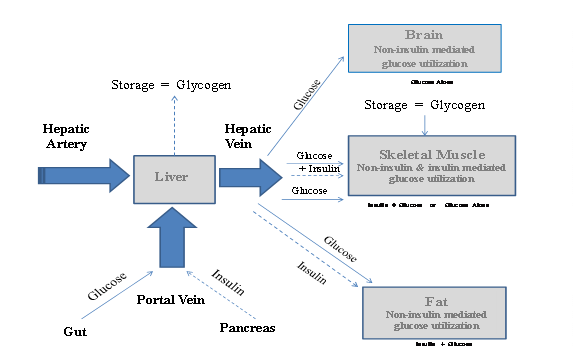
Insulin release is mediated by a variety of activating or inhibitory triggers including nutrients, neurotransmitters, chemical signals and drugs. (Martens 2009, Torres 2009) Glucose enters pancreatic beta cells via glucose transporters (GLUT 2) and is metabolized to ATP. (Ashcroft 1999, 2006, Jensen 2008, Thorens 2001) The relative excess of ATP compared to ADP results in the closure of potassium ATP channels and thus produces cellular membrane depolarization. This depolarization opens voltage-dependent channels and permits extracellular calcium influx. Higher levels of calcium in the cytosol result in insulin exocytosis. (Hou 2009, Weiderkehr 2008)
Insulin release can occur both 1) in response to glucose exposure or mixed meals; and 2) in background oscillation patterns present even during the fasted state. (Goodner 1982, Lefebvre 1987, Porksen 1995, 1997, Weigle 1987) Like other peptide hormones it is secreted in pulses.(Carmel 1976, Dierschke 1970, Goodner 1977, Lang 1979, 1982, Laursen 1995, Stagner 1980, Shapiro 1988, Tannenbaum 1976) Background insulin secretion occurs in high frequency pulses (8-15 minute intervals) and ultradian (recurrent cycles within a 24 hour period) pulses. (Goodner 1977, Lang 1979, Polonsky 1986, 1988, 1998 NEJM), Sturis 1992, Song 2000)
Classically, insulin release was thought to occur in two segments: first-phase, which is a short secretory burst occurring within minutes of a stimulus, and second-phase, which is longer in duration and whose magnitude reflects the extent of stimulus. (Caumo 2004, Cherrington 2002, del Prato 2002. Henquin 2002) First-phase insulin release is diminished in patients with Type 2 diabetes and may be impaired in subjects at risk for the development of Type 1 diabetes. (Cutfield 2004, Fujita 1975, Grendal 2007, Porte 1991, Ratzmann 1981, Smith 1988, Vialettes 1988) As the technologic capability to detect hormone pulsations has improved, it has become apparent that the classic two-phase release reflects an extreme response to a large and sudden glucose load. Under more physiologic stimulus conditions than an intravenous glucose bolus, the occillatory pattern of insulin release changes, but is not as discrete. (Caumo 2004, Lefebvre 1987, Matthews 1991, Polonsky 1995, 1998, Porksen 2002)
Insulin pulsatility can vary by amplitude, inter-pulse interval, and regularity of periodicity. Abnormalities in the background insulin oscillations are present in patients with Type 2 diabetes, in high risk relatives of patients with Type 2 diabetes, and in obese patients with insulin resistance. (Hollingdal 2000, Hunter 1996, Juhl 2001, Lang 1981, O’Rahilly 1988, Radetti 1998, Zarkovic 1999) These abnormal pulsatile patterns can be normalized by weight loss. (Radetti 1998, Zarkovic 2000) Conversely, a pattern of the normal number of pulses, but lower insulin content per pulse, is present in persons engaged in endurance training. (Engdahl 1995) Curiously, although there appears to be a correlation between the insulin pulsatility frequency and peripheral insulin sensitivity, a similar relationship is not present for hepatic insulin sensitivity. (Hunter 1996)
Some investigators have focused on the response of the liver to insulin in the portal venous system. Indeed, it is known that hepatic enzymes involved in glucose metabolism, e.g., hepatic glucokinase (hexokinase IV), phosphofructokinase, and pyruvate kinase require higher insulin levels (200-500 µU/ml) than can be achieved by absorption of subcutaneous insulin into the peripheral venous system. (Aoki 1992, Basu 2000, Clark [Saltiel, Pessin] 2007, Vester 1963) Furthermore, some, but not all, investigators observed increased hepatic extraction of insulin during endogenous insulin pulsations. (Grubert 2005, Meier 2005) It is thought that this entrainment (synchronization) of hepatic insulin receptor turnover optimally suppresses endogenous hepatic glucose output and determines the amount of insulin to be delivered to the peripheral circulation. These findings prompted some researchers to consider insulin treatments which more closely replicate the normal physiologic state and increase the amount of portal vein and hepatic exposure to insulin. Although peritoneal or portal vein delivery could achieve this goal, these routes of administration are very invasive and impractical. Intravenous administration can approximate these levels of hepatic exposure to insulin only if systemic venous insulin levels (and the risks of hypoglycemia) are also high. Nonetheless, some investigators have proposed these alternative routes of insulin administration.
Diabetes and standard insulin therapy
In the following section, we describe the typical route of administration (subcutaneous injection), along with some additional routes of administration that are under development, because all of these are generally self-administered, unlike intravenous insulin. We then describe the typical inpatient settings in which IV insulin infusion might be transiently employed and the types of monitoring that are required for safe administration.
Because insulin and insulin analogues are proteins, they are degraded and/or denatured in the digestive tract. For this reason, the majority of insulin products are given parenterally, i.e. via routes that do not involve the gastrointestinal (GI) tract. The standard outpatient route of administration is a) subcutaneous injection via syringe or pen-cartridge device; or b) subcutaneous infusion from an external pump (usually programmable with bolus and basal rates). (Bohannon 1999, Pickup 1997, Saudek 1997) In patients on peritoneal dialysis, insulin may be added to the dialysate (dialysis fluid). (Chan 1993, Tzamaloukas 1991) Although less invasive methods of insulin administration (inhaled, buccal, or oral) were or are under investigation, patients can safely self-administer insulin via the standard, albeit more invasive, subcutaneous (or intramuscular) injections. ( C&E News, DOC, Drugs.com; FDA Dear Doctor Letter, Heller 20007, Khafagy 2007, Royle 2004 with Cochrane update 2008, Yadav 2009)
There are, however, circumstances under which insulin therapy is administered intravenously (IV) by trained medical personnel. Intravenous insulin is used to treat patients with serious or life-threatening metabolic disturbances, e.g., diabetic ketoacidosis and hyperosmolar coma, other conditions limiting the level of consciousness, and/or rapidly fluctuating nutritional needs and intake. (Braithwaite 2003, Furnary 2003, Harrower 1979, Kitabchi 2003, Knapke 1989, Lazar 2000, Malmberg 1997, Park 1992, Pezzarossa 1988, Piters 1977, Pomposelli 1998, Scott 1999, Van den Berghe 2003,Woolfson 1981, Zerr 1997) This route of insulin administration is employed under these types of circumstances because the onset of IV insulin action is rapid and the half-life IV insulin is short; permitting rapid therapeutic intervention in response to rapidly changing clinical conditions. (Turnheim 1988)
When such IV insulin therapy is administered, it is carried out in an inpatient hospital setting such as in the intensive care unit (ICU), the surgical suite, or the step-down unit where the capacity to intensely monitor patients with professional-grade clinical laboratory testing (glucose and electrolytes including potassium levels) and skilled nursing staff are present and enhance safety. (Braithwaite 2004, FDA pump guidance, Grissinger 2003, Hellman 2004, Joint Commission 2000, Paice 1986) The duration of IV insulin therapy is short; patients are switched to sustainable, less invasive, less risky treatment, i.e., insulin injections, other parenteral hypoglycemic drugs, oral hypoglycemic agents, and/or diet, when their condition stabilizes. (Laveria 2008)
When insulin is delivered intravenously, additional precautions are also undertaken during administration because the therapeutic index (the safety margin as expressed by the lethal dose divided by the therapeutic dose) is very narrow when given by this route. (Braithwaite, Grissinger 2003, Hellman 2004) In addition to the potential lethality of hypoglycemia, patients are at risk for hypokalemia because of the intracellular flux (flow from the blood into the cells) of potassium with insulin administration. (Bergman, Rave 1999, Simmons 1994, Tattersall 1999) Hypokalemia itself can be lethal because it precipitates cardiac rhythm disturbances. (Alfronzo, Cohn 2000) Intravenous insulin is administered as part of a diluted solution along with adjunctive potassium. The insulin product typically is diluted to a fixed concentration, e.g., 1 unit insulin/1 cc of normal saline, which is piggy-backed onto the maintenance IV line. (Joint Commission 2000, Novolin insulin label) This IV line flows through an adjustable rate pump for precise dose control. A concomitant IV potassium solution is also piggy-backed into the infusion system. (Novolin insulin label) Doses of insulin, potassium, and hydrating fluids are separately titrated based on frequent results from professional-grade clinical laboratory tests (and not home-use monitoring systems). (Diabetes Today 2009, FDA-Gaines Meter Error, FDA Glucose Meter Alerts, FDA Glucose Meter Advisory Committee Meeting 2001, FDA Meter Review Criteria, Hovorka 2006, Kost 1998, Meguro 2005, Perera 2009) In all cases, the insulin is given as a daily replacement therapy. The doses may be similar to normal physiologic needs or may reflect the increased requirements of insulin resistance. The insulin is not administered intermittently as an adjunctive metabolic stimulant.
Diabetes and metabolic measurements
In the following section, we delineate the typical role of respiratory gas measurements, and the respiratory quotient (RQ) measurement in particular, in medical care and physiologic assessment. We do this because serial outpatient respiratory quotient values are sometimes employed in OIVIT. Metabolism can be determined by measurements of inspired O2 (oxygen) and expired O2 and CO2 (carbon dioxide). (Bartlett 1952, Fuji 2003, Jéquier 1987) The respiratory exchange ratio (RER) (CO2 elimination/O2 absorbed across alveolar capillary membranes) is the same as the RQ (CO2 production/O2 utilization) under steady state (equilibrium) conditions. The respiratory quotient of completely oxidized substrates is represented by: the number of carbon atoms in the fuel molecule divided by the number of carbon atoms plus the number of hydrogen atoms divided by four and minus the number of oxygen atoms divided by 2. Complete metabolism of pure carbohydrate substrate yields a RQ of 1.0 whereas complete metabolism of fat yields a respiratory quotient of approximately 0.7. The RQ associated with proteins is mid-range and is complicated by a variety of metabolic pathways. In other words, the value of the RQ reflects the extent to which a food substrate is oxidized and the composition of that food substrate. The accuracy and/or utility of this RQ ratio depends on a number of factors including a) the absence of substrate other than carbohydrate, fat and protein, b) substrate disappearance only due to oxidation (and not the urinary losses of glucose or protein that can be present in diabetes), c) the rested state, and d) the fasted state. (Felber 1977, 1980, Golay 2002, Meyer 1980, Schutz 1983) Furthermore, there are errors in the measured metabolic rate that are introduced when the urinary urea nitrogen (UUN) component is not included in the calculation. (Mansell 1990) Typically these measurements are obtained through indirect calorimetry (measurement of heat [energy] using respiratory gases) using metabolic carts (an instrument that measures respiratory gases). Some determinations such as resting energy expenditure (REE) may require the use of additional equations (Fleisch, Harrison-Benedict, or Reed) and patient data. The carts must be regularly calibrated. (Diamond 2007, Hopkins 2003 Policy 159) All devices may not provide comparable data. (Webster 1999, Wells 1998) Typically, RQ measurement is limited to the inpatient setting. Frequently, the measurements are used in the adjustment of nutrition and ventilation parameters. (AARC 2004) Such measurements would not be taken more often than once daily.
There are RQ related measures, Basal Metabolic Rate (BMR) and REE, which are more typically obtained in the outpatient setting. (Compher 2006, Henry 2005) The former is the energy expenditure in the rested, fasted (10-12 hrs), thermally neutral state. The latter does not require the same degree of fasting. BMR (kcal/24 hrs) ~ REE (kcal/24 hrs) = 5.68 VO2 (ml/min) + 1.59 VCO2 - 2.17 UUN (g/24 hrs) = 5.46 VO2 + 1.75 VCO2. These are used in weight management and weight loss programs. Serial measurements are seldom required. Other measurements, such as VO2 max, are obtained during exercise for cardiopulmonary or fitness testing. (ATS/ACCP 2003, Diamond 2007, Guimaraes 2008) Multiple determinations within a 24 hour period are seldom required.(ATS/ACCP letter 2009)
By contrast, the advocates of OIVIT interpret the RQ as a measure of carbohydrate metabolic efficacy in diabetic patients and further utilize RQ measurements to assess the response to the insulin infusion and to adjust subsequent insulin infusion doses. (See OVIT Development below.)
Outpatient Intravenous Insulin Therapy (OIVIT) Development
OIVIT is a regimen with assorted components which may include insulin, insulin infusion, infusion pump use, metabolic cart measurements, and laboratory testing along with an insulin dosing regimen that is either an explicit algorithm or a more implicit dosing schedule (Figure 2). In OIVIT, insulin is intravenously administered in the outpatient setting to patients to patients who are not critically ill. Most commonly, it is delivered in pulses, but it may be delivered as a drip solution. (ADRI, Diabetes.net, MI, Normedex, Pulse Medix, Strategic Partners-Bionica, VitalCare.) In contrast to conventional therapeutic regimens, the insulin administration is adjunctive to the patient’s routine diabetic management regimen (oral agent or insulin-based) or other disease management regimen, typically performed on an intermittent basis (often weekly), typically delivered as a series of fixed duration infusions on a single day, and frequently performed on a perpetual basis without duration limits. (ADRI) Glucose or other carbohydrate is available ad libitum (in accordance with patient desire).
Because the insulin infusions reportedly serve, not as hormone replacement therapy, but as a metabolic stimulator, the accompanying metabolic measurements play a somewhat different role in OIVIT therapy. The RQ, which may or may not include a measured UUN component, is claimed to be used as a determination of the patient’s carbohydrate oxidative efficacy. In addition, it is claimed to be used to assess the patient’s metabolic response to the insulin infusion and to adjust subsequent insulin infusion doses via an explicit or implicit algorithm to achieve an RQ of 0.9 to 1.0. It is not known how adjustments are made for the confounding ad libitum (in accordance with patient desire) consumption of glucose or other carbohydrate to reduce hypoglycemia. As many as six serial (pre-, post-) RQ measurements are being obtained during a single treatment session. Blood or serum glucose levels are obtained to reduce the risk of hypoglycemia and/or to adjust subsequent insulin infusion doses via an explicit or implicit algorithm. The precision and accuracy of the methodology used to assess blood/serum glucose levels and the frequency of assessment are not well delineated. (N.B. Personal or home- use glucose monitors do not have the same level of precision as professional point of care diagnostic devices or laboratory chemistry.) (Diabetes Today 2009, FDA-Gaines Meter Error, FDA Glucose Meter Alerts, FDA Glucose Meter Advisory Committee Meeting 2001, FDA Meter Review Criteria, Hovorka 2006Kost 1998, Meguro 2005, Perera 2009) It is not known whether other parameters, e.g., serum triglycerides, and lipid/fat accumulation, are monitored. (See Insulin Delivery)(Aguis2009)
Some OIVIT business plans describe/propose the use of closed or semi-closed loop systems for administration in the home setting. (Strategic Partners-Bionica) Blood is to be intermittently aspirated by the infusion pump. Glucose levels are to be detected by sensors and could be monitored remotely. Insulin is to be delivered from commercial cartridges and not via standard diluting solutions. (See FDA Status.)(See NCD Manual 40.3 regarding closed blood glucose control device in the NCD section.)
Claimed benefits for this therapy include 1) improved glycemic control without increased hypoglycemia or with reduced hypoglycemia; 2) improved blood pressure control; 3) decreased progression of nephropathy; 4) reversed autonomic neuropathy (postural hypotension, abnormal diurnal blood pressure, hypoglycemic unawareness); 5) improved wound healing and reduced amputation risk; 6) reduced perception of disability; and 7) improved quality of life. (ADRI, Aoki patent series , Logan-Darroguh 1995) Other claims involve use of the therapy in the peri-islet cell transplant period and use in non-diabetic patients. (Aoki patent series, MI, Mirbolooki 2009, Normedex) Furthermore, it has been claimed that no adverse events have ever been reported for this treatment. (Strategic Partners-Bionica, Normedex)
The physiology underlying this therapy was studied extensively in Europe, but this line of investigation was abandoned there in the early 1990s after a series of conflicting study results. (See Exploratory Studies in Humans.) Of note, respiratory quotients were not used for either dosing or efficacy assessment in the European studies. This is in contrast to their use with the subsequent commercialization of OIVIT in the U.S. (ADRI, MI, Strategic Partners-Bionica, VitalCare, Pulse Medix,Normedex, Diabetes.net, SEC Files) The initial proponents of this therapeutic regimen were Dr. Thomas Aoki and colleagues. To further study the regimen and treat patients, the Aoki Diabetes Treatment Institute (ADRI) was founded in 1986. (ADRI) Dr. Aoki holds patents for metabolic activation therapy for a variety of diabetic and non-diabetic indications. (Aoki patent series) Respiratory quotient measurements are integral to his proprietary treatment regimen, which is available at ADRI and other authorized institutions. (ADRI, ADRI Injunction, MI, Strategic Partners-Bionica, VitalCare, Pulse Medix, Nomedex, CA Cases ) A variety of other clinics provide similar therapy. (ADRI, MI, Strategic Partners-Bionica, VitalCare, Pulse Medix, Nomedex, Diabetes.net)
Figure 2: Elements of OIVIT
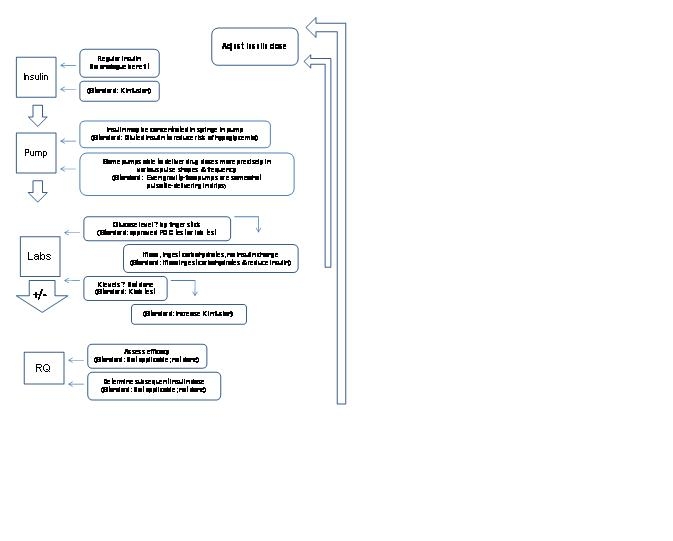
Analogue = insulin analogue
K = potassium
? = possibly
POC = point of care
RQ = respiratory quotient
OIVIT could involve computer input of data and/or dosing algorithm.
Exploratory animal studies
There have been three important animal studies in which IV insulin therapy was employed. We include them as background as they illustrate the preliminary research in this field. One study was a short-term physiology study. Two studies employed IV insulin as a chronic treatment. In the latter two studies, the IV insulin therapy replaced standard therapy; it was not used as intermittent adjunctive therapy.
Grubert et al. studied 15 fasted non-diabetic mongrel dogs with the 3.5 hour glucose clamp. (Grubert 2005) Endogenous insulin was suppressed with somatostatin. Glucagon was kept constant. Sampling catheters were placed in the portal and hepatic veins as well as the aorta. Infusion catheters were placed in the splenic and jejunal veins (a physiologic location). The dogs underwent three infusion procedures: constant insulin infusion (1 µU/kg/min, pulsatile insulin (12 µU/kg/min over 1 minute every 12 minutes), and pulsatile insulin (3 µU/kg/min over 4 minutes every 12 minutes). The results indicated that hepatic glucose uptake did not differ by the infusion mode or by the amplitude and duration of the insulin pulse when the pulses were given at 12 minute intervals (physiologic for canines).
Weigle et al. studied five streptozoticin-induced diabetic baboons over three approximately month long consecutive treatment periods (pulsatile insulinà continuous insulinà pulsatile insulin). (Weigle 1991) Insulin needs throughout the day were determined and then delivered either continuously or as pulses every 10 minutes. (N.B. This differs from the use of intravenous insulin as adjunctive therapy.) Endpoints included four-times daily glucose values, HbA1c, fasting hepatic glucose production (via titrated glucose dilution) and beta-cell function (via response to glucose and arginine loads). Counter-regulatory hormones and other metabolic parameters were also measured. There were no differences by treatment group. Of note, glucagon secretion, which is postulated to contribute to the putative glycemic control associated with pulsatile insulin, was not entrained by insulin. (Matthews 1983, Paolisso 1987, 1988)
Koopmans et al. studied 25 streptozoticin-induced diabetic rats in parallel treatment groups (pulsatile insulin, continuous insulin, control) for approximately 18 days. (Koopmans 1996) Endpoints included fasting glucose levels, fasting insulin levels, diurnal glucose areas-under-the-curve, diurnal insulin areas-under-the-curve and urinary glucose levels. Counter-regulatory hormones, insulin binding, glucose uptake by adipocytes and glycerol production by adipocytes were also measured. Glycemic control was markedly improved as was the anti-lipolytic action of insulin (as measured by glycerol production) in the setting of pulsatile insulin.
Results from animal studies assessing pulsatile IV insulin have been contradictory. The reasons for the disparate animal results are not well understood. The baboons had a higher level of endogenous insulin reserve (comparable to some patients with Type 2 diabetes) whereas the dogs had endogenous insulin suppressed by somatostatin and the rats had a lower insulin level (comparable to Type 1 diabetes). It is possible that higher levels of endogenous insulin obscure small insulin pulses or that longer periods of infusion are required or that the biologic benefit conferred by pulsatility is relatively small in comparison to other environmental factors. (Grubert 2005, Koopmans 1996, Schmitz 1986, 1994, Wiegle 1991)
No animal studies have demonstrated that exogenous IV insulin therapy results in improved glycemic control via activation of hepatic enzymes, e.g., hepatic glucokinase (hexokinase IV), phosphofructokinase, and pyruvate kinase. Hexokinase IV has been studied via glucokinase activators and by genetic over-expression. (Ajius 1995, 2009, Coughlan 2008, Gunn 1973, Hariharan 1997, Mateo 1989, Matschinsky 2006, Pal 2009, Payne 2007, Postic 2001, Soane 1996, Takeuchi 1996, Tilley 2009) In a rodent model using titrated doses of the hexokinase IV gene in a viral vector, even gene activity that was three-times the normal did not result in euglycemia. (O’Doherty 1999) Other studies have suggested that activation of this enzyme could result in hypertriglyeridema and pathologic lipid deposition. (O’Doherty 1999, Pal 2009)
III. History of Medicare Coverage
The outpatient intravenous insulin therapy regimen has several constituent components which are discussed below. We also note other items and services related to other uses of insulin to make clear to the reader that they do not pertain to OIVIT.
A. National Coverage Determinations
There were no prior national coverage determinations on the outpatient intravenous insulin therapy regimen as an integrated program or on its components when furnished pursuant to an outpatient intravenous insulin therapy regimen.
Medicare has a national coverage determination on Glucose Testing as a diagnostic laboratory test, at 190.20 of the NCD Manual. It includes the following among its indications and limitations:
"Blood glucose values are often necessary for the management of patients with diabetes mellitus, where hyperglycemia and hypoglycemia are often present. They are also critical in the determination of control of blood glucose levels in the patient with impaired fasting glucose (FPG 110-125 mg/dL), the patient with insulin resistance syndrome and/or carbohydrate intolerance (excessive rise in glucose following ingestion of glucose or glucose sources of food), in the patient with a hypoglycemia disorder such as nesidioblastosis or insulinoma, and in patients with a catabolic or malnutrition state. In addition to those conditions already listed, glucose testing may be medically necessary in patients with tuberculosis, unexplained chronic or recurrent infections, alcoholism, coronary artery disease (especially in women), or unexplained skin conditions (including pruritis, local skin infections, ulceration and gangrene without an established cause).
Many medical conditions may be a consequence of a sustained elevated or depressed glucose level. These include comas, seizures or epilepsy, confusion, abnormal hunger, abnormal weight loss or gain, and loss of sensation. Evaluation of glucose may also be indicated in patients on medications known to affect carbohydrate metabolism.
Effective January 1, 2005, the Medicare law expanded coverage to diabetic screening services. Some forms of blood glucose testing covered under this national coverage determination may be covered for screening purposes subject to specified frequencies. See 42 CFR 410.18 and section 90, chapter 18, of the Claims Processing Manual, for a full description of this screening benefit."
We note for the reader’s convenience the Home Glucose Monitors NCD at 40.2 of the NCD Manual. We are not making a determination on home glucose testing with this decision. Diagnostic testing of glucose levels is distinguished from home glucose testing, which is covered as durable medical equipment (DME).
We note only for the reader’s convenience that Medicare has a national coverage determination on Closed-Loop Blood Glucose Control Device (CBGCD) as an inpatient hospital service at 40.3 of the NCD Manual. However, OIVIT as described is not the same as CBGCD. It includes the following indications and limitations:
The closed-loop blood glucose control device (CBGCD) is a hospital bedside device designed for short-term management of patients with insulin dependent diabetes mellitus (Type I). It consists of a rapid on-line glucose analyzer; a computer with a controller for the calculation and control of the infusion of either insulin or dextrose; a multi-channel infusion system; and a printer designed to record continuous glucose values and to provide cumulative totals of the substances infused. Its primary use is for the stabilization of Type I diabetics during periods of stress, such as trauma, labor and delivery, and surgery, when there are wide fluctuations in blood sugar levels. It serves to temporarily correct abnormal blood glucose levels (hyper- or hypo-glycemia) and this correction is made by infusion of either insulin or dextrose. Its use is generally limited to a 24- to 48-hour period because of potential complications; (e.g., sepsis, thromboses, and nonportability, etc.). The CBGCD requires specialized training for use and interpretation of its diagnostic and therapeutic contribution and continuous observation by specially trained medical personnel. Use of the CBGCD is covered for short-term management of insulin dependent diabetics in crisis situations, in a hospital inpatient setting, and only under the direction of specially trained medical personnel.
We also note only for the reader’s convenience that Medicare has a national coverage determination on Infusion Pumps at 280.14 of the NCD Manual. However, this addresses the subcutaneous administration of insulin, not the IV administration.
B. Benefit Categories
Because Medicare is a defined benefit program, an item or service must fall within a benefit category as a prerequisite to Medicare coverage: 1812 (Scope of Part A); 1832 (Scope of Part B); 1861(s) (Definition of Medical and Other Health Services); of the Social Security Act.
1. Insulin and Insulin Infusion
Drugs and biologicals and the administration of drugs and biologicals may be considered to be within the benefit category of the Social Security Act section 1861(s)(1), physicians’ services; section 1861(s)(2)(A), services and supplies (including drugs and biologicals which are not usually self-administered by the patient) furnished as incident to a physician’s professional service; and section 1861(s)(2)(B), hospital services (including drugs and biologicals which are not usually self-administered by the patient) incident to physicians’ services rendered to outpatients.
2. Metabolic Cart/Respiratory Quotient
The metabolic testing may be considered a benefit under the benefit category set forth in Title XVIII of the Social Security Act, section 1861(s)(3) (diagnostic tests - other), a Part B benefit.
IV. Timeline of Recent Activities
March 25, 2009: The CMS opened an internally generated National Coverage Analysis (NCA) to evaluate the available evidence for outpatient intravenous insulin treatment, as well as the devices used to administer the therapy and the laboratory monitoring and medical-nursing surveillance required for implementation in the various outpatient settings, and the role for accompanying metabolic testing (including respiratory quotients). The initial 30-day comment period began.
April 24, 2009: The initial 30-day public comment period closed; 187 timely comments were received.
August 31, 2009: CMS met with Dr. Thomas Aoki, Mr. Bruce Parsons and Mr. Dick Costigan from the Aoki Institute.
September 25, 2009: CMS posts the Proposed Decision Memorandum and opens a 30 day public comment period on the proposed decision.
V. Food and Drug Administration (FDA) Status
The outpatient intravenous insulin treatment (OIVIT) regimen under evaluation consists of multiple elements; some of which are intrinsic to the regimen and others which are optional: a biological pharmaceutical agent, an intravenous infusion device, glucose monitoring device, a metabolic cart for the determination of respiratory quotients, and a treatment regimen. The pump may have the capacity to be programmed for the delivery of insulin pulse patterns. The pump may have an integrated glucose monitoring device. The pump may be linked to a computer. A computer may integrate information about glucose levels, prior drug responsiveness, and respiratory quotients as well as other variables. The treatment regimen for OIVIT may be explicitly codified as a treatment algorithm or nomogram or may be more implicit. None of the proposed elements for OIVIT have specifically been approved by the FDA for OIVIT. There are no approved computer systems for the input and integration of glucose and respiratory quotient data for either the determination of an insulin dose or for the determination of an insulin dose linked to a pumping mechanism. The FDA has not approved this treatment regimen as a comprehensive unit.
Although none of the elements used in OIVIT have been specifically approved by the FDA for the OIVIT regimen, regular human insulin, which has long been used off-label for intravenous drips in the in-patient setting, especially intensive care units, did acquire a labeled indication for intravenous use (Novolin R in 2005). On-label use, however, requires adequate monitoring:
INDICATIONS "Novolin R may be administered intravenously under proper medical supervision in a clinical setting for glycemic control";
DOSAGE AND ADMINISTRATION "Intramuscular and intravenous administrations of Novolin R are possible under medical supervision with close monitoring of blood glucose and potassium levels to avoid hypoglycemia and hypokalemia. For intravenous use, Novolin R should be used at concentrations from 0.05 U/ml to 1.0 U/ml in infusion systems with the infusion fluids 0.9% sodium chloride, 5% dextrose, or 10% dextrose with 40 mmol/l potassium chloride using polypropylene infusion bags."
Two insulin analogues insulin, insulin aspart (Novolog) and glusisine (Apidra), which have more rapid onset of action when absorbed through the skin, but not when delivered via intravenous or peritoneal routes, have also acquired FDA approval for the intravenous administration indication. Their labels include similar language regarding the medical supervision, laboratory monitoring, and drug dilution required for safe use.
VI. General Methodological Principles
When making national coverage determinations under section 1862(a)(1)(A) of the Social Security Act, CMS evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that an item or service falling within a benefit category is reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member. The critical appraisal of the evidence enables us to determine to what degree we are confident that: 1) the specific assessment questions can be answered conclusively; and 2) the intervention will improve health outcomes for patients. An improved health outcome is one of several considerations in determining whether an item or service is reasonable and necessary.
For diagnostic testing, CMS generally considers evidence in the hierarchical framework of Fryback and Thornbury (1991) where Level 2 addresses diagnostic accuracy, sensitivity, and specificity of the test; Level 3 focuses on whether the information changes the physician's diagnostic thinking; Level 4 concerns the effect on the patient management plan and Level 5 measures the effect of the diagnostic information on patient outcomes. Most studies have focused on test characteristics and changes in physician diagnostic thinking and have not considered health outcomes, such as mortality or morbidity. We believe that health outcomes are more important than test characteristics.
A detailed account of the methodological principles of study design that the agency utilizes to assess the relevant literature on a therapeutic or diagnostic item or service for specific conditions can be found in Appendix A. In general, features of clinical studies that improve quality and decrease bias include the selection of a clinically relevant cohort, the consistent use of a single good reference standard, and the blinding of readers of the index test and reference test results.
Public comment sometimes cites the published clinical evidence and gives CMS useful information. Public comments that give information on unpublished evidence such as the results of individual practitioners or patients are less rigorous and therefore less useful for making a coverage determination. CMS uses the initial public comments to inform its proposed decision. CMS responds in detail to the public comments on a proposed decision when issuing the final decision memorandum.
VII. Evidence
1. Questions
We are providing a summary of the evidence that we considered during our review. This section presents the agency's evaluation of the evidence considered for the assessment questions.
- Is the evidence sufficient to conclude that an outpatient intravenous insulin therapy (OIVIT) regimen improves health outcomes in Medicare beneficiaries?
In order to answer question 1, we asked the following questions about the individual components that comprised OIVIT.
- Do outpatient insulin treatment strategies that incorporate diagnostic respiratory quotient (RQ) testing to guide intravenous insulin therapy improve health outcomes compared to strategies that do not use RQ testing?
- Do outpatient insulin treatment strategies that incorporate diagnostic urine urea nitrogen (UUN) testing to guide intravenous insulin therapy improve health outcomes compared to strategies that do not use UUN testing?
- Do outpatient insulin treatment strategies that incorporate diagnostic blood glucose or potassium testing to guide intravenous insulin therapy improve health outcomes compared to strategies that do not use blood glucose or potassium testing?
- If the answer to any of the above questions is affirmative,
- Which health outcomes of Medicare beneficiaries are improved?
- What is the duration of therapy required to effect a clinically significant improvement and how durable is that improvement (in the presence or absence of continued therapy)?
- Which patient characteristics reliably predict a clinically significant favorable or unfavorable health outcome?
Outcomes of greatest interest
Medicare is most interested in therapeutic modalities that have been shown to improve morbidity and mortality, i.e., hard clinical endpoints, in its beneficiaries. Reliance on intermediate (or surrogate) outcomes, such as change in test results, can be misleadingly encouraging.
For example, early studies in diabetes management led to hypotheses that hyperglycemia itself was the cause of diabetic complications such as neuropathy, retinopathy and macrovascular coronary artery disease. Thus, researchers focused on the improvement of glycemic control as a goal or desired outcome of treatment.
Indeed, the Diabetes Complications and Control Trial (DCCT) demonstrated that glycemic control could blunt the onset/progression of microvascular retinal and renal complications in Type 1 diabetic patients. (DCCT 1993) The United Kingdom Prospective Diabetes Study (UKPDS) suggested the same in Type 2 diabetic patients, but also indicated that the patient management of Type 2 diabetes with its impaired insulin action in combination with other metabolic defects involved more than insulin replacement. (UKPDS 33, 34 1998)
The subsequent studies by the Veterans’ Administration (VA) and the National Institutes of Health (NIH) have shown that intensive glycemic control using oral hypoglycemic medications and insulin does not confer major protection against cardiovascular disease and may increase morbidity and mortality..(ACCORD Gerstein 2008, Skyler 2009, VA Abraira 1997, 2003, VA Duckworth 2009) In addition, glycemic control has not been shown to reverse end-stage microvascular diabetic complications in either Type 1 or Type 2 patients and renal disease in Type 2 patients, when its underlying etiology is macrovascular or hypertensive in nature. (Orchard 2006)
Health outcomes of interest include improvements in the following morbidities of diabetes: retinal disease, microvascular renal complications, macrovascular renal disease and cardiovascular disease. Intermediate outcomes such as glycemic control, blood pressure control and blood pressure medication use are not accorded evidentiary weight for the reasons discussed above, as they can be misleading. Treatment burden and adverse effects, particularly severe hypoglycemia and any potential side effects attributable to the metabolic changes induced by the outpatient intravenous regimen of insulin administration such as alterations in serum lipids and lipid deposition in tissue are of interest because of the advanced age and co-morbid conditions present in many Medicare beneficiaries.
Health disparities
The Medicare beneficiary population includes several subgroups of diabetic patients. The largest segment (95+%) comprises older Type 2 diabetic patients (age 65+ years), whose primary cause of death is cardiovascular disease. Minority populations are overly represented in this diabetic population. Younger beneficiaries with Type 2 diabetes frequently have co-morbid conditions, e.g., psychiatric disease, which limit diabetes management. Younger beneficiaries with Type 1 disease are frequently in the Medicare program because they are already disabled by diabetes-related complications. As such, Medicare is interested in treatment modalities that address these complications and clinical conditions. (MCAC 2006)
2. External technology assessments (TAs)
CMS did not commission an external TA for this NCA. We are aware of two external assessments of outpatient intravenous insulin treatment. We describe them below briefly.
1. Blue Cross/Blue Shield-California (BCBS 2001)
The therapeutic regimen was reviewed on February 14, 2001. The following was extracted from the assessment:
"Other alternatives to PIVIT exist for treatment of blood sugar, blood pressure, and kidney disease. Intensive insulin therapy has been documented to produce more sustained effects on glycemic control and antihypertensive therapy (particularly with ACE [angiotensin converting enzyme] inhibitors) to produce excellent control of blood pressure adequate to reduce or prevent progression of diabetic nephropathy. Based on the two randomized, controlled trials, it is difficult to conclude that PIVIT improves net health outcomes as much as or more than the established alternatives. Finally, the substantial drop-out rates in the randomized, controlled trials suggest that maintaining the schedule of weekly PIVIT in addition to daily intensive insulin needed to achieve its potential benefits may be difficult for many patients under conditions of usual medical practice. Therefore, TA criteria 2-5 are not met.
RECOMMENDATION It is recommended that pulsatile intravenous insulin therapy does not meet Blue Shield TA criteria for patients with type 1 diabetes mellitus."
2. Hayes (A proprietary technology assessment and rating firm in Landsdale, PA) (Hayes 2007) This therapeutic regimen was initially reviewed July 14, 2006 and subsequently updated August 15, 2007 and September 29, 2008. The following was extracted from the Executive Summary:
"Important questions regarding CIIIT are:
- Does CIIIT improve glycemic control and/or reduce incidence or progression of sequelae of diabetes, compared with conventional intensive insulin therapy?
- Have definitive patient selection criteria for CIIIT been established?"
"Major complications of CIIIT have not been reported.
Definitive patient selection criteria for CIIIT have not been established.
Conclusions: There is insufficient evidence from the limited number of published studies to conclude that CIIIT is effective in reducing symptoms, improving glycemic control, or preventing diabetic sequelae in patients with type 1 diabetes. Although results of several of the studies suggest that CIIIT may improve glycemic control, facilitate blood pressure control, and/or slow progression of nephropathy, the lack of adequate controls, randomization, and blinding, and the small sample sizes of the available studies preclude definitive conclusions regarding the health benefit of CIIIT. Double-blind RCTs of adequate size are necessary to evaluate this therapy adequately."
3. Internal technology assessment
CMS staff conducted a comprehensive search of materials to address the clinical questions delineated above. CMS staff extensively searched Medline (1965 to present) for primary studies evaluating pulsatile insulin and intravenous insulin therapy. The emphasis was on studies structured to assess long-term efficacy and adverse events. CMS staff likewise searched the Cochrane collection, the National Institute for Health and Clinical Excellence (United Kingdom) appraisals, and the Agency for Healthcare Research and Quality (United States) library for systematic reviews and technology assessments. Systematic reviews were sought to help locate any obscure publications and abstracts.
The CMS reviewed FDA reviews of the registration trials for intravenous insulin, intravenous pumps, glucose testing, and indirect calorimeters as well as FDA safety data for intravenous pumps and insulin. CMS staff reviewed the transcripts from the FDA Advisory Committee meetings on glucose monitoring systems, the warnings on glucose monitoring systems and the guidance document on 510k clearance for external infusion devices. CMS staff reviewed the 2008 NIH/FDA workshop proceedings about closed-loop insulin infusion systems. CMS searched the National Institutes of Health (NIH) Clinical Trials.gov database for ongoing/completed trials of outpatient intravenous insulin therapy. We used internet searches to identify websites with clinical trial results and/or pump information and/or respiratory quotient measurement information, press releases for clinical trials and/or pump information and calorimetry devices and U.S. government regulatory action. Preference was given to English language publications. Keywords used in the searches included: intravenous-insulin, pulsatile-insulin, hepatic activation, metabolic activation, calorimetry, respiratory quotient, metabolic cart, infusion pump and insulin pump.
We reviewed external technology assessments, evidence based guidelines, professional society position statements and public comments. We conducted an internal technology assessment of pertinent animal studies, exploratory physiologic studies in humans and longer-term clinical studies in patients. In addition, we reviewed eight trials listed in ClinicalTrials.gov.
Published Studies: Exploratory Human Physiology Studies
There have been multiple physiologic studies of pulsatile insulin. (See Figure 3 and Table 1) They have been of short-term duration with testing occurring during treatment or immediately after 1-2 days of treatment. Normal subjects, Type 1 diabetic patients, and Type 2 diabetic patients have been tested. Similar to the animal studies discussed in the Background section, these studies used exogenous insulin as replacement for endogenous insulin and not as adjunctive therapy.
Figure 3: Published Human Physiology Studies: Pulsatile Insulin
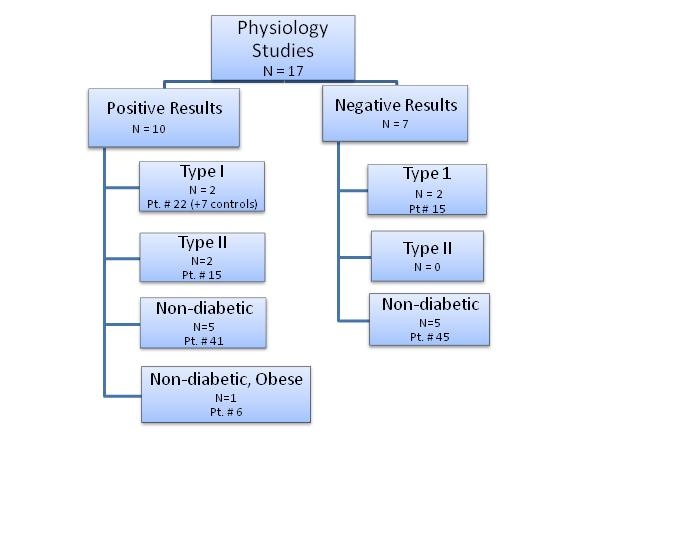
Table 1: Published Studies of Pulsatile IV Insulin: Exploratory and Physiologic: Human
| Study/ Yr Published/ Funding |
Endpoints |
Patient Type/ Diabetes Type/ Number/ Other Features |
Blinding |
Randomization |
Control |
Duration |
|
Related StudiesContinuous Insulin
|
|
|
|
|
|
|
|
Foss
1982
NIH
Howard Hughes |
Glucose clamp-related parameters
Glucose control p glucose & mixed meal loads
RQ p glucose & mixed meal loads (not for dosing)
à better glucose control |
Type 1/non-DM control
Poor glycemic control
No complications
N=5/5
Continuous 72 hr Biostator infusion w less rigid blood glucose parameters than in a euglycemic clamp |
No |
No |
Pre- & post tx
Non-DM |
4 d |
|
Aoki
1983
NIH |
Glucose clamp-related parameters Forearm glucose extraction RQ (not for dosing)
à better splanchnic glucose extraction (indirect) |
Type 1
N=9
Biostator infusion w less rigid blood glucose parameters than in a euglycemic clamp |
No |
No |
Pre- & post tx |
4 d |
|
Positive Results |
|
|
|
|
|
|
|
Matthews 1983 |
Insulin binding to monocytes
Glucose levels
à better glycemic control
à more receptor binding
|
Non-diabetic
N=9 (but 3 DCed) Somatostatin suppression of endogenous insulin used; glucagon replaced |
No |
Random order of tx arms |
2 tx arms
(continuous vs pulsatile) |
2 d |
|
Bratusch-Marrain 1986 Fonds zur Forderung der Wissenschaff-lichen Forschung Osterreichs |
Glucose clamp parameters (tritiated glucose)
à less HGO for same dose or more suppression w lower doses
à suppression more evident with duration of dosing |
Type 1
Study A n=8
Study B n=5 |
No |
No |
2 tx arms
(continuous vs pulsatile P)
A= 60% insulin delivery w P
B=equal total insulin doses |
1 d each infusion |
|
Schmitz 1986 Aarhus University Research Council Institute of Experi-mental Clinical Research Danish Diabetic Association Danish Medical Research Council |
Glucose clamp parameters (tritiated glucose) No RQ used
à better metabolic clearance, but not lower HGO (perhaps bc of higher insulin doses) & more evident after 3.5 hrs
à GH & glucagon same |
Non-diabetic N=8 Higher doses of insulin than Paolisso 1991 |
No |
Random order of tx arms |
2 tx arms
(continuous vs pulsatile) |
1 d each infusion (longer 6 hr clamps) Separated by 2-4 wks |
|
Paolisso 1988 |
Glucose clamp parameters No RQ used
à higher glucose infusion rate in the last hour of infusion
à improved lipids |
Type 2 (no drug) N=8 Somatostatin suppression of endogenous insulin used; glucagon replaced |
No |
Random order of tx arms |
2 tx arms
(continuous vs pulsatile) |
I d each infusion |
|
Paolisso 1988 |
Glucose clamp parameters Beta cell response to arginine
à more C-peptide suppression in normals
à glucagon response to arginine altered w pulsatility |
Type 1/non-DM control
N=9/7 |
No |
Random order of tx arms |
2 pt types Pre- & post tx
2 tx arms
(continuous vs pulsatile)
Different insulin doses |
1 d each infusion |
|
Paolisso 1990 |
Glucose clamp parameters (tritiated glucose)
à higher glucose infusion rate in the last hour of infusion
à improved lipids |
Non-diabetic elderly N=7 Somatostatin suppression of endogenous insulin used; glucagon replaced |
No |
Random order of tx arms |
2 tx arms
(continuous vs pulsatile |
1 d each infusion |
|
Ward 1990 National Health & Medical Research Council of Australia Royal Australasian College of Physicians Kellion Diabetes Fdn Novo Labs |
Minimal model parameters (tritiated glucose)
Insulin binding to monocytes Glucagon & other hormones NEFA No RQ used
à more insulin sensitivity
à binding reduced
à fewer NEFA |
Non-diabetic (non-obese) N=8 3 late day meals given |
No |
No |
Pre- & post tx Comparison to prior continu-ous infusion |
1 d |
|
Paolisso 1991 |
Glucose clamp parameters hepatic glucose output (tritiated glucose) No RQ used
à less HGO w q13 min pulse |
Non-diabetic (nl wt) N=9 Somatostatin suppression of endogenous insulin used; glucagon replaced |
No |
Random order of tx arms |
3 tx arms (continuous vs pulses q 13 min vs pulses q 26 min |
1 d x3 separated by 1+ wk |
|
Paolisso 1992 Fonds de la Recher-che Scientifique Medical of Belgium Fonds de la Recher-che Facultaire of Liege |
Glucose clamp parameters
(tritiated glucose) Glucagon
à improved metabolism ~ to 33% more continuous infusion insulin |
Type 2 Failed oral agents N=7 Somatostatin suppression of endogenous insulin used; glucagon replaced |
No |
Random order of tx arms |
3 tx arms (continuous 88 U/kg vs pulsed 88 U/kg vs pulsed vs 117 U/kg |
1 d x3 separated by 5+ d |
|
Schmitz 1994 | Glucose clamp parameters
(tritiated glucose) Glycerol GH & glugcagon RQ over 30 min-not for tx (urine urea collected for protein oxidation) Tissue NEFA Tissue glycogen synthase Tissue LPL
à HGO same
à ? glucose disposal same
à Glycogen synthase same
à Suppressed glycerol & lipid oxidation
à >LPL
à > Glucose oxidation |
Non-diabetic (obese F) N=6 Somatostatin suppression of endogenous insulin used |
No |
Random order of tx arms |
2 tx arms
(continuous vs pulsatile) |
1 d each infusion (longer 6 hr clamps) Separated by 2-4 wks |
|
Verdin 1984 Fonds National de la Recherche Scientifique Fonds de la Recher-eche Scientifique Medical of Belgium |
Glucose clamp parameters glucose infusion rate metabolic clearance rate; hepatic glucose output (tritiated glucose) No RQ used
à clamp parameters not different |
Non-diabetic (nl wt) N=7 No somatostatin suppres-sion Higher insulin doses than Kerner |
No |
Random order of tx arms |
2 tx arms
(continuous vs pulsatile) |
1 d x2 separated by 1+ wk |
|
Negative Results |
|
|
|
|
|
|
|
Paolisso 1986 |
Glucose clamp parameters (tritiated glucose) Glucagon levels
à glucose turnover not affected |
Non-diabetic N=9 Somatostatin suppression of endogenous insulin used; glucagon replaced |
No |
No |
2 tx arms
(continuous vs pulsatile) |
1 d x2 separated by 1 wk |
|
Paolisso 1987 |
Glucose levels
à Any hypoglycemic effect w pulsatile insulin was most evident at lower glucagon levels |
Type 1 N=6 Somatostatin suppression of endogenous insulin used; glucagon replaced at various levels |
No |
Random order of tx arms |
6 tx arms (continuous vs pulsatile + 3 glucagon infu-sion rates) |
1 d x3 |
|
Kerner
1988 |
Glucose clamp parameters
à glucose infusion not different |
Non-diabetic (nl wt) N=7 Somatostatin suppression of endogenous insulin used |
|
Random order of tx arms |
2 tx arms (continuous vs pulsatile) |
|
|
Heinemann*
1989
Baxter Travenol |
Glucose clamp parameters RQ including UUN (not for dosing) Glucose control after SQ insulin during exercise Glucagon level
à worse glucose control |
Type 1/non-DM control Good glycemic control No complications N=9/3 |
No |
No |
Pre- & post tx 2 tx arms (continuous vs pulsatile) Non-DM |
1 d x2 w/in 1 wk |
|
Paolisso 1989 Fonds de la Recher-che Scientifique Medical of Belgium Fonds de la Recher-che Facultaire of Liege Italian Government |
Glucose clamp parameters
(tritiated glucose) Glucagon No RQ used
à glycemic control not better
à endogenous glucose output high & driven by glucagon especially w pulsatile insulin |
Non-diabetic (nl wt) N=6 Somatostatin suppression of endogenous insulin used; glucagon replaced |
No |
No |
4 tx arms (insulin contin-uous or pul-satile w gluca-gon continuous or pulsatile) |
1 d x4 separated by 1+ wk |
|
Ward 1989 |
Minimal model parameters (tritiated glucose) Glucagon NEFA No RQ used
à suppressed HGO in all
à more glucagon suppression
à not better glycemic control |
Non-diabetic (non-obese) N=6 |
No |
Random order of tx arms |
3 tx arms (continuous x2 vs pulsatile > continuous vs pulsatile > continuous) |
Paired infusions on single day |
*Aoki was co-author
Levy-Marchal 1983 paper reviewed
but excluded because was SQ not IV.
Foss 1993 paper reviewed, but excluded
because was SQ not IV.
Pasolisso 1990 reviewed, but excluded
because was pulsatile glucagon. |
D=Day
DCed=Discontinued
F=Female
GH=Growth Hormone
HGO=Hepatic glucose output
LPL=Lipoprotein lipase
NEFA =Non-esterified fatty acids | Nl=normal
RQ=Respiratory Quotient
Tx=Treatment
Wk=Week
W=With |
Published Studies: Longer Clinical Trials
Clinical studies have been conducted by two groups of investigators: Aoki et al. and Dailey et al. (See Figure 4 and Table 2) Weinrauch et al. have studied a subset of the Dailey patient population more extensively. Unlike the physiology studies, these studies used exogenous insulin as replacement for endogenous insulin and not as adjunctive therapy. Non-standard endpoints were used in many of the studies. (Figure 4 and Table 3)
Figure 4: Clinical Studies of Outpatient Intravenous Insulin Therapy (OIVIT)
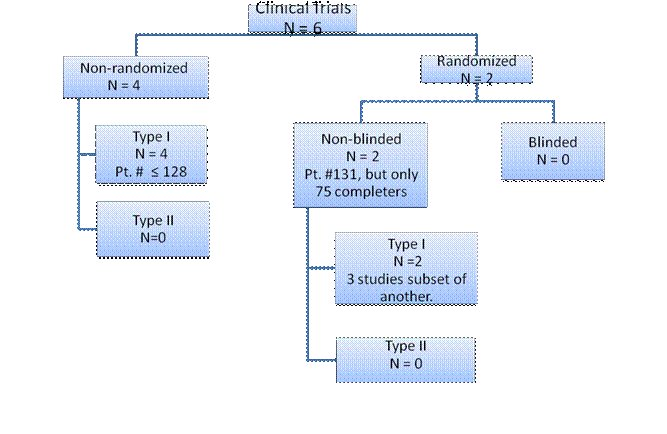
Table 2: Published Studies of Pulsatile IV Insulin: Extended Use: Human
| Study/ Yr Published/ Funding |
Endpoints |
Patient Type/ Diabetes Type/ Number/ Other Features |
Blinding |
Randomization |
Control |
Duration |
Aoki
1993
AMSYS |
Glucose Control
Hypoglycemia |
Type 1
N= 20
Refractory Glucose Control
Often complications |
None |
No |
Pre-Tx |
Not fixed
7 to 71 mo |
Aoki
1995A
AMSYS
B-M Corp |
BP Rx Dose |
Type1
N= 41 > 32 > 26
(Subset of Aoki 1995C) |
Not stated |
Yes |
X-over |
3 mo each arm |
Aoki
1995B
AMSYS |
Orthostatic BP
Glucose Control |
Type 1
N= 3
Refractory hypotension |
Not stated |
No |
Pre-Tx |
3 mo |
Aoki
1995C
AMSYS
B-M Corp |
Diurnal BP
Glucose Control |
Type 1
N= 74 |
Retrospective |
Retrospective
Pooled data & selected from reportedly randomized , prospective trials, but all 1o
sources cannot be located
(See Aoki 1995A)
|
Yes |
3 mo |
Aoki
1999
AMSYS
B-M Corp |
Renal Function
Glucose Control |
Type 1
N= 31 |
None |
No |
Pre-Tx |
Not fixed
12 to 84 mo |
Dailey
2000
AMSYS |
Renal Fxn
Glucose Control |
Type 1
N= 90à 71à 49
Mild to moderate renal dysfunction |
None |
Initially yes, but duration changed |
Yes |
Initially 12 mo
Later 18 mo |
Weinrauch
2007
ADTC
Pat Covelli |
Glucose Control
Renal Function
BP & BP Rx
Cardiac Mass & Fxn
Neuropathy
Hemostasis
Other Labs |
Type 1
N= 18
Mild to moderate renal dysfunction
(Subset of Dailey 2000) |
Test reader only |
Larger group yes, but is subset |
Yes |
12 mo |
Weinrauch
2009
ADTC
Pat Covelli |
DMIS Survey
Renal Fxn |
Type 1
N= 19
Mild to moderate renal dysfunction
(Subset of Dailey 2000) |
Not stated |
Larger group yes, but is subset |
Yes |
12 mo |
Weinrauch
2009C
ADTC
Pat Covelli |
Perception of disability with DMIS survey & subsets |
Type 1
N= 19
Mild to moderate renal dysfunction
(Subset of Dailey 2000) |
Not stated |
Larger group yes, but is subset and treatment groups pooled for survey analysis |
Yes |
12 mo |
ADTC= Advanced Diabetes Treatment Center
B-M Corp= Boehringer Mannheim Corporation
BP= blood pressure
DIMS= Diabetes Impact Management Scale |
Fxn= function
Rx= medication
Yr= year |
|
Table 3: Randomized Clinical Studies of outpatient IV Insulin Therapy by Endpoint
| Endpoint |
Type 1 diabetes |
Blind |
Glycemic Control |
Type 2 diabetes |
Blind |
Glycemic Control |
Glycemic Control |
YES (Dailey 2000)
HbA1c change in pts w mild to moderate renal dysfunction
Change in glycemic control not compared between groups. Appears not to differ.
N= 49 (Only 71 of 90 entrants completed 12 mo and only 49 completed 18 mo.) (Intent-to-treat analyses not performed.) |
NO |
NOT better |
NO |
|
|
| Acute Complications |
| Hypoglycemia |
NONE |
|
|
NONE |
|
|
| Hypoglycemic Awareness |
NONE |
|
|
NONE |
|
|
| Chronic Complications |
| Retinopathy |
NONE |
|
|
NONE |
|
|
| Nephropathy |
YES
(Dailey 2000) Creatinine clearance change in pts w mild to moderate dysfunction Change at 12 mo not significant. Change at 18 mo was significant. N= 49 (Only 71 of 90 entrants completed 12 mo and only 49 completed 18 mo.) (Intent-to-treat analyses not performed.) |
NO |
NOT related to glycemic control |
NONE |
|
|
Neuropathy-Autonomic |
NONE |
|
|
NONE |
|
|
Neuropathy-Sensory |
NONE |
|
|
NONE |
|
|
Cardiovascular Disease |
NONE |
|
|
NONE |
|
|
Islet Transplant Survival |
NONE |
|
|
Not Applicable |
|
|
| Other |
Rx Reduction |
YES (Aoki 1995A)
Blood Pressure Rx Dose Change
6 mo cross-over study
N= 26 (Only 32 of 41 recruits entered study. Only 26 completed.)
(Intent-to-treat analyses not performed.) |
NO |
NOT better
NOT related to glycemic control |
NONE |
|
|
Cognition |
NONE |
|
|
NONE |
|
|
Other |
|
|
|
|
|
|
QOL |
A subset (Weinrauch 2009) of a "randomized’ study (Dailey 2000)
12 mo time point data.
DIMS survey for disability
24% survey data missing; survey modified
N= 19 |
NO |
NOT better
NOT related to glycemic control |
NONE |
|
|
DIMS= Diabetes Impact Management Scale.
IV= Intravenous
Mo= Month |
QOL= Quality of Life
Rx= Medication
W= With |
DC’d= Discontinued |
There have been two semi-independent groups of researchers in this field. We have categorized the studies by research group and subcategorized the studies by topic or design features.
Aoki et al. Studies
Most of the studies conducted by Aoki and colleagues have been case series or retrospective studies without contemporaneous controls. (Tables 1, 2.) (Aoki 1993, 1995b, 1995c, and 1999)
Non-randomized Studies
Glycemic Control/Hypoglycemia
The first study assessed a series of 20 Type 1 diabetic patients with highly variable self-test glucose measurements (undefined) and/or frequent hypoglycemia (undefined). Reportedly, most patients had a chronic diabetic complication. Glycemic control was poor (mean HbA1
c 8.5 %); the mean insulin dose was low (34 units/day) despite attendance at a specialty clinic for one or more years and a four-injection daily insulin regimen. Subjects were followed for 7 to 71 months. Reportedly, glycemic control improved. HbA1
c values from discrete time points, however, were not presented. Rather the change in HbA1
c over time using the method of least squares was employed although there were too few data points to establish linearity for three subjects. Reportedly, glycemic control improved (HbA1
c 7%) without an increase in the daily insulin dose or frequency of hypoglycemia. The authors concluded that
"….the absence of a control group requires that the data presented here be interpreted cautiously".
Hypotension
This study was followed by a report of three patients with Type 1 diabetes and refractory orthostatic hypotension (undefined) who were treated with weekly pulsatile insulin for three months. (Aoki 1999c) Patients were assessed with positional blood pressure measurements (regimen defined), ambulatory blood pressure measurements (diurnal time intervals for measurements not defined), tilt table testing, and cardiac autonomic function testing (NDX device from Q Med; paired testing done in two patients), and glycemic control. The orthostatic change appears to be smaller in two subjects; one with modest antecedent and continuing glycemic control (HbA1c ~ 7.7%) and one with poor control and marked improvement (HbA1c from 9.5 to 7.5%). The authors suggest that all patients improved and that glycemic control contributed to "amelioration of autonomic neuropathy" and high dose insulin may have improved vasoconstriction. The authors report that discontinuation worsened "postural symptomatology", but did not provide positional blood pressure readings.
Renal Function
This study was followed by a report of a three-center review of 31 patients with Type 1 diabetes and overt nephropathy (persistent albuminuria > 300 mg/24 hours, but creatinine clearance > 15 ml/min) and treated with four daily injections of insulin. (Aoki 1999) All subjects had received weekly pulsatile insulin for at least one year. Mean values of the changes in HbA1c, creatinine clearance and urinary protein were calculated. Exit endpoint values, and not endpoint values from discrete time points, appear to have been used. No correlative analyses of the change in glycemic control and the change in renal function were presented. Although mean glycemic control improved (HbA1c 8.6 to 7.6%), mean urinary protein and creatinine clearance did not change. Despite the absence of a control, the authors suggested that "the minimal decline in creatinine clearance during the observation period clearly indicates that stabilization or arrest of progression of the overt diabetic neuropathy in our study patient cohort". They further inferred that pulsatile insulin treatment is effective despite treatment duration although they suggested that it might be most effective in the early months of treatment. There was no discussion of whether there was any imbalance in the populations who received treatments for different time periods. There was no discussion of the potential importance of treatment duration as a study variable and how this should be addressed in future studies.
Diurnal Blood Pressure
In a 1995 study by Aoki et al. (Aoki 1995b), the authors state that it is a prospective, randomized three-month trial, which is retrospective only because it was a post hoc analysis of patients pooled from multiple studies. Some patients (number unknown) from a randomized trial with two 3-month crossover treatment arms and a total of 26 patients (n= 52 paired treatments). (Aoki 1995a) The source of the remainder of the patients remains unknown. A search of Medline does not reveal the primary publication of any other randomized trials by this author group. The abstract of the 1995b publication suggests that some of the patient data may have been derived from an uncontrolled case series (n= 20) (Aoki 1993), but even this does not account for all participants. (See segment 5 of the 1995c abstract.) Reportedly this study was done to assess diurnal patterns in blood pressure. Insulin doses and blood pressure medications (ACE inhibitors, calcium channel blockers, loop diuretics, and alpha-agonists) and doses were adjusted during the pre-treatment stabilization period and during the treatment period(s).
The methods section does not include any information on blood pressure measurement including types, positions, devices, frequency, and time-frame definition of night-time vs daytime (and whether such definitions were delineated a priori). Patients without hypertension (approximately 23%) or normal diurnal blood pressure profiles were not excluded. Patients were not stratified on the basis of potentially important variables such as glycemic control, renal disease severity, presence of hypertension prior to renal disease onset, severity of nocturnal hypertension, and presence of autonomic neuropathy. Only limited mean data results were reported. The authors reported a statistically significant, although biologically small decrease in HbA1c (0.5%) within the unblinded experimental treatment arm, but did not perform comparative statistics between the two treatment groups. They presented only mean diurnal blood pressure ratios and the temporal changes in the ratios and did not clarify the statistical analyses used on these derived parameters. The use of ratios obscures the primary data and its variability. They did not present the mean diurnal blood pressure measurements and the temporal changes in mean diurnal blood pressure. No correlative analyses between blood pressure change and glycemic control change were presented. The authors concluded that pulsatile insulin treatment prevented further deterioration in the circadian blood pressure pattern and suggested in both this publication and in a 2001 review that the changes were mediated through improvements in autonomic neuropathy. (Aoki 2001)
Randomized Studies (Aoki et al.)
Anti-hypertensive Medication Use
The only randomized study of pulsatile insulin by Aoki and colleagues assessed the change in blood pressure medication use after three months in a cross-over study of patients with Type 1 diabetes and hypertension, but with a creatinine clearance > 15 ml/min.(Aoki 1995a) Anti-hypertensive medication doses were assigned equivalency units. Patients were not given an absolute hypertensive medication score. Rather, dose requirements "…were compared to a baseline value and expressed as a percentage change". Investigators attempted to maintain stable blood pressure values. The authors reported that pulsatile insulin use resulted in a 46% reduction in individual patient anti-hypertensive medication use and suggested that the likelihood of medication discontinuation was greatest in those with the least severe renal disease, fewest baseline medication requirements, and shortest duration of hypertension. The study was not blinded. There were no published power analyses. There were no intent-to-treat analyses. There was notable attrition since only 32 of the 41 enrolled subjects completed the stabilization period (one to three months). Another six were excluded from the per-protocol completer analysis. The mean treatment arm data suggest that during each of the pulsatile insulin phases, the systolic blood pressure readings increased 5 to 10 mm Hg. Assessments of paired t= 0 and t= 3 month treatment arm blood pressure changes for individuals were not presented.
Dailey et al. Studies
The studies conducted by Dailey et al. and Weinrauch et al. are related. (Figure 4, Table 2) The Weinrauch study population(s) are a subset of the Dailey population. (Figure 4, Table 2)
Randomized Studies (Dailey, et al.)
Annualized Creatinine Clearance
The Dailey study recruited Type 1 diabetic patients with creatinine clearances between 30 and 80 ml/min and albuminuria > 100 mg/d. Prior diabetes control with intensive insulin therapy with multiple daily injections and dietary management was not required. Such measures were instituted for patients desiring study entry. The training regimen was in place for a minimum of four weeks, but the time duration was not uniform. No information on the number of entrants to the training regimen was provided.
No information on the prior use of intensive insulin-dietary therapy and the duration of pre-enrollment training and subsequent randomization was presented. Ultimately, 90 subjects from seven centers were enrolled. Presumably the enrollment was 1:1, but this was not stated. Only the treated patients were infused so there was no subject blinding. No null hypothesis was presented (Tables 2 and3). No power analyses were presented. The methods section only briefly described the insulin therapy and referenced the 1993 Aoki paper. Laboratory assessments of glycemic control (HbA1c) and renal dysfunction (serum creatinine, blood urea nitrogen, serum protein, 24-hour protein (albumin), 24-hour urine collection for creatinine clearance) were performed along with blood pressure (systolic-sitting, diastolic-sitting, and mean arterial) and anti-hypertensive medication (% using ACE inhibitors) assessments. Extensive cardiac (left ventricular mass, left ventricular function), autonomic neuropathy (heart rate variation, diurnal blood pressure variation), and hemostatic assessments as well as radionucleotide determination of glomerular filtration rate (GFR), surveys, and other laboratory tests (lipids, advanced glycated end products) were performed at one or more of the centers. (Dailey 2001; Weinrauch 2007, 2009a, 2009b) Seventy-one subjects completed the 12 month study, but it is not known how these subjects were distributed by treatment arm. There was no intent-to-treat analysis. Indeed, there was no complete analysis for those who had completed the 12 month study because the change in an important variable, creatinine clearance, was not statistically significant.
The study was extended for another six months. Forty-nine subjects (54% of enrollees) (experimental treatment arm n= 23; control arm n= 26) completed 18 months of study. Reportedly, the inconvenience of weekly clinic visits was the major reason for drop-out. Again, there was no intent-to-treat analysis. There was an analysis of 18-month completers (Table 4). There was no declared plan with statistical penalties for early/multiple looks at the data and multiple endpoints (Tables 3 and 4). Changes in creatinine clearance were calculated, adjusted to a 12 month basis, and compared to patients in the 12 month cohort, but it is not clear as to whether the 18 month cohort members were included in the 12 month cohort calculations because they were not excluded in the manuscript’s Table 1.) The authors report that annualized changes in creatinine clearance were statistically significant (2.21± 1.62 vs 7.79± 1.88 ml/min; p= 0.03) and assert that pulsatile insulin treatment reduces progression of diabetic nephropathy independent of glycemic control, ACE inhibitor treatment or blood pressure control. The values of these variables at entry and exit (18 month), their change and the statistical assessments were not clearly presented. The potential imbalance of glycemic control at baseline (albeit not statistically significant because of small numbers) was not addressed. There were no assessments of respiratory quotient values and treatment efficacy.
Table 4: Variables Important for Outcomes in Completers of 18 Months of Dailey Study
| Study Variables |
Control
t=0 mo |
Control
t=18 mo |
Experimental
t=0 mo |
Experimental
t=18 mo |
|
HbA1c (%) |
9.13 |
8.19 |
8.61 |
7.68 |
Systolic Blood Pressure (mm Hg) |
133.0 |
- |
133.6 |
- |
Diastolic Blood Pressure (mm Hg) |
79.2 |
- |
76.9 |
- |
Mean Arterial Blood Pressure (mm Hg) |
97.1 |
98.6 |
96.7 |
95.3 |
ACE Inhibitors (% patients) |
77 (Unclear if entry or exit) |
- |
70 (Unclear if entry or exit) |
- |
Other Antihypertensive Agents (% patients) |
- |
- |
- |
- |
Serum Creatinine (mg/dl) |
1.66 |
- |
1.50 |
- |
24-hour Creatinine Clearance (ml/min) |
59.6 |
- |
55.3 |
- |
24-hour Urine Protein (mg/d) |
2107 |
2609 |
2057 |
2362 |
24-hour Urine Albumin (mg/d) |
- |
- |
- |
- |
Weinrauch et al. Studies
The Weinrauch populations appear to constitute a subset of the Dailey population.
Subsets of a Randomized Population (Weinrauch et al.)
Glycemic Control/Creatinine Clearance/Echocardiographic Findings
In the earliest study (2007) there were 18 patients whereas in the latter of the studies (2009a & b) there were 19 patients. This discrepancy was unexplained. Unlike the study above, analyses were conducted after 12 months of treatment. In the 2007 study of the role cardiovascular mechanisms might play in renal disease progression of pulsatile insulin treated diabetic patients, the blind was limited to only readers of study tests. No null hypothesis was presented (Tables 2 and 3). No power analyses were presented. There was no declared plan with statistical penalties for multiple endpoints. The analyses appear to be limited to those who completed the 12 month study and not to all entrants, i.e., intent-to-treat. There appears to be a problem with patient number accounting. In the experimental treatment group, 17% of patients were reported to have edema. This would not be possible with the n= 10 denominator. Echocardiographic measurements and functional assessments, autonomic function, and hemostatic laboratories were not thought to have changed during the study or to differ between treatment arms. However, there appears to be baseline imbalance or a trend to baseline imbalance in mean arterial pressure (MAP), an important variable in a study assessing the role of OIVIT and cardiovascular factors on renal dysfunction. Control 103.8±3.4 vs. experimental 96.2±2.7mmHg. (Weinrauch 2007) . This imbalance favors the OIVIT intervention arm. There was a similar imbalance for glycemic control.
Glycemic control improved for the study population as a whole. Indeed glycemic control may have been better and improved more in the control arm although statistical significance may have not been reached because of small sample size: Control t0= 9.8± 0.5% and t12 mo= 8.0± 0.3%; Experimental t0= 9.1± 0.6% and t12 mo= 8.5± 0.6%. Absolute creatinine clearance and the change in creatinine clearance reportedly did not differ by treatment group: Control t0= 55.4± 7.0 ml/min and t12 mo= 45.8± 7.0 ml/min; Experimental t0= 58.4± 7.0 ml/min and t12 mo= 55.4± 8.7 ml/min. Statistical significance may not have been reached because of small sample size, but even if the values were statistically significant, their biological significance and whether they can be extrapolated over time is uncertain. No correlation calculations between changes in glycemic control and changes in renal function with corrections for baseline renal function and baseline glycemic control were presented. No correlation calculations between renal function and carbohydrate oxidation were presented. Reportedly, RQ values increased immediately after infusions 0.854 to 9.54 in the initial weeks vs 0.826 to 0.915. There was no explanation as to why the baseline level relative carbohydrate oxidation appears to have decreased over the course of treatment and why the response to treatment appears to have declined over the course of therapy. Despite these findings and questions, the authors assert that pulsatile insulin with its improved fuel efficiency is responsible for preservation of renal function.
Author Modified Self-report Disability Survey
In subsequent analyses, the authors used the Hammond-Aoki Diabetes Impact Management Scale (DIMS) (44 self-report questions-six point scale) to assess its relationship with renal function and glycemic control (Hammond 1992; Weinrauch 2009a) and to measure perceived disability (Weinrauch 2009b) although it is not clear that the treatment contributed to better renal function as measured by creatinine clearance or improved glycemic control as measured by HbA1c. The authors did not comment on the validity of using quality-of-life testing in groups in which the treatment was not blinded. The authors did not indicate whether such a survey was validated for longitudinal data or only cross-sectional data. (Watkins 2004) The authors acknowledged that up to 24% of survey response data could be missing for individual respondents. They addressed this by data interpolation; mean treatment group data for a question was substituted for the respective missing data. It is not clear as to whether data were excluded because intent-to-treat analyses were not performed. The authors did not present the comparative results of the DIMS for the treatment groups (baseline values, exit values, 12 month exit values, change in values [12 month completers], change in values [intent-to-treat]). The authors did report that longitudinal changes in DIMS (if any) did not correspond to changes in renal function or glycemic control.
The investigators reformatted that data and conducted additional analyses. They analyzed each survey question for statistically significant change and then excluded questions in which no longitudinal change was found. They reclassified the remaining questions into "emotional" and "physical" responses (Table 5). They determined that the five "physical" questions indicated neurologic status. They then compared scores of the 12 question DIMS subset, the seven question "emotional", and the five question "physical" subset with various study parameters. They reported that "changes in the five physical questions... correlated with stable creatinine, stable creatinine clearance, and decrease in left ventricular hypertrophy" although there appears to have been post hoc determination of the renal function and left ventricular mass categories and patients were pooled regardless of treatment status—suggesting that the study was more an assessment of the abbreviated/subcategorized DIMS test than of the therapeutic intervention. (Weinrauch 2009a) Nonetheless, they also reported that "pulsatile intravenous insulin, when added to standard multiple-dose insulin therapy, was demonstrated to improve subjective perception of neurologic disability…" on the basis of the unblinded testing results of the five question "physical" subset. (Weinrauch 2009b) Although such survey data are typically interpreted and validated as a unit, the authors did not provide validation for these changes. Nor did they provide substantiation (by physical exam or other testing) for the "physical" questions. There was no declared plan with statistical penalties for multiple endpoints. Neither study clarified a role for respiratory quotient testing.
Table 5: Selected Subset of the Hammond-Aoki Diabetes Impact Management Scale
| Author Categorization of Questions |
Question |
|
|
During the past month: |
Emotional |
How much time were you lacking enough energy? |
|
How well have you slept? |
|
How often have you been able to function well in your usual occupation? |
|
Have you participated in and enjoyed family life? |
|
Have you eaten what you wanted to? |
|
Have you felt depressed? |
|
Have you felt optimistic about your diabetes? |
Physical |
Have you been bothered by blurring of vision? |
|
Did burning, tingling, pain, or numbness bother you in your hands? |
|
Have you been bothered by feeling faint/dizzy on sitting/standing up? |
|
How often did you have diarrhea? |
|
How often were you able to function sexually as well as you wanted? |
Other Studies
We did not find published studies on the utility of pulsatile insulin in wound healing and amputation risk reduction.
We did not find published studies of pulsatile insulin in the peri-whole pancreas transplant or peri-islet transplant period despite published speculation about its utility. (Mirabolooki 2009)
4. Medicare Evidence Development and Coverage Advisory Committee (MEDCAC)
A MEDCAC meeting was not convened for this issue.
5. Evidence Based Guidelines
Numerous agencies and professional groups (Agency for Healthcare Research and Quality [AHRQ], American Association of Clinical Endocrinologists [AACE], American Association for Respiratory Care [AARC], American College of Cardiology [ACC], American College of Chest Physicians [ACCP], American College of Physicians [ACP], American Diabetes Association [ADA], American Dietetic Association [ADA], American Geriatrics Society [AGS], American Heart Association [AHA], American Thoracic Society [ATP], Canadian Diabetes Association [CDA], Endocrine Society, Juvenile Diabetes Research Foundation [JDRF], Scottish Intercollegiate Guidelines Network [SIGN], and Veterans Health Administration [VHA]) were queried about guidelines regarding intravenous insulin therapy and metabolic cart measurements. Many of these groups have a variety of guidelines for diabetes, nutrition, and cardiopulmonary disease management.
We found no evidence-based guidelines addressing the use of outpatient intravenous insulin therapy, the use of calorimetry to direct dosing or monitor intravenous insulin therapy in any type of patient, and/or the use of calorimetry in diabetic patients. Only the CDA addressed intravenous insulin and did so in the "In-hospital" chapter. "Role of Intravenous Insulin Infusion. Intravenous (IV) insulin infusion therapy should be considered during critical illness, or other illness requiring prompt glycemic control, or prolonged fasting (NPO status) (7). IV insulin infusion therapy should be administered only where frequent blood glucose (BG) monitoring and close nursing supervision are possible. Staff education is a critical component of the implementation of an IV insulin infusion protocol. IV insulin protocols should take into account the current and previous BG levels (and, therefore, the rate of change), and the patient’s usual insulin dose."
6. Professional Society Position Statements
American Association of Clinical Endocrinologists (AACE)
We received the following statement from the American Association of Clinical Endocrinologists (AACE).
In 2004, an AACE task force addressed this issue and submitted a report to the AACE Board of Directors which essentially determined, after an extensive review of available data, that no definitive long-term benefit from intermittent intravenous insulin therapy could be identified. The AACE Board of Directors approved the Task Force’s report and, since that time, has not seen any study or other evidence that would cause the Association to readdress the issue.
American Diabetes Association (ADA)
The ADA issues positions statements, expert committee reports, workgroup reports, technical reviews, and consensus statements. Upon review, none of these documents address outpatient intravenous insulin, whether pulsatile or not. None of these documents address the use of calorimetry and respiratory quotients in managing diabetic patients.
- Position Statements
(http://care.diabetesjournals.org/content/32/Supplement_1/S98.full.pdf+html; Accessed 6/15/09)
- Committee Reports and Consensus Statements
(http://care.diabetesjournals.org/content/32/Supplement_1/S96.full.pdf+html; Accessed 6/15/09)
- Technical Reviews
(http://care.diabetesjournals.org/content/32/Supplement_1/S95.full.pdf+html; Accessed 6/15/09)
- ADA Diabetes Care: Insulin Administration ADA Position Statement
Diabetes Care, Vol 27, Suppl 1, Jan 2004: S106-S-109.
(http://care.diabetesjournals.org/content/27/suppl_1/s106.full.pdf+html; Accessed 6/15/09)
- Nutrition Recommendations and Interventions for Diabetes: A Position Statement of the ADA
(http://care.diabetesjournals.org/content/31/Supplement_1/S61.full.pdf+html; Accessed 6/15/09)
Several years ago, the ADA was asked to assess the pulsatile insulin therapeutic program. The presented data were found not to be persuasive. (Communication with Richard Kahn, Ph.D., Scientific Director; September 2009)
Endocrine Society
Since the publication of the proposed decision, the Endocrine Society has adopted a position supporting noncoverage of OIVIT. See Vigersky letter [PDF, 75KB].
Department of Defense
There are no centrally determined positions on insulin therapy. Pulsatile insulin therapy does not appear to have any/any significant use in the Army (Dr. Robert Vigersky; Walter Reed; personal communication March 2009) or the Navy (Dr. Patrick Clyde; National Naval Medical Center-Bethesda; personal communication March 2009)
Indian Health Service
Outpatient insulin therapy does not appear to have any/any significant use in the Indian Health Service (Dr. Susan Karol, chief medical officer for the IHS; personal communication August 2009)
Veterans’ Health Systems
There are no centrally determined positions on insulin therapy. Pulsatile insulin therapy does not appear to have any /any significant use in the VA system (Dr. Leonard Pogach, Director-VA New Jersey Healthcare System, Center for Healthcare Knowledge Management; personal communication March 2009)
7. Expert Opinion
We received the following from David M. Harlan, M.D., Chief, Diabetes Branch, National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health
At Dr. Koller’s request, I have reviewed the claims pertaining to pulsatile intravenous insulin delivery’s ability to reverse diabetes complications, or to improve clinical islet transplantation outcomes, whether that pulsatile insulin is delivered in the peri-operative period, in the post surgical period, or for rescue therapy. I am unaware of any data I consider scientifically credible to justify those claims.
Please feel free to contact me should you have questions.
See full text at
http://www.cms.gov/Medicare/Coverage/DeterminationProcess/downloads/id231Harlan.pdf [PDF, 32KB].
8. Public Comments
Public Comment Period: March 25, 2009 – April 24, 2009
Public comment sometimes cites the published clinical evidence and gives CMS useful information. Public comments that give information on unpublished evidence such as the results of individual practitioners or patients are less rigorous and therefore less useful for making a coverage determination. The CMS uses the initial public comments to inform its proposed decision. The CMS responds in detail to the public comments on a proposed decision when issuing the final decision memorandum.
During this initial public comment period, the CMS received a total of 187 comments from individuals/groups. The majority (n= 134) were patient testimonials about their own outpatient intravenous insulin treatment; three were testimonials in support of family members who had received outpatient intravenous insulin therapy. The remaining comments came from non-physician health care professionals (n= 21), physician clinicians (n= 17), academic physicians (n= 2); an attorney (n= 1), a health insurance industry representative (n= 1), and assorted or unspecified contributors. The majority favored CMS coverage of outpatient intravenous insulin therapy; five did not; two provided non-specific comments. Six provided literature citations and/or other materials with their comments but no new scientific publications were uncovered. Full text comments without personal health information can be viewed at: http://www.cms.gov/mcd/viewpubliccomments.asp?nca_id=231.
Public Comment Period on the Proposed Decision: September 25, 2009 – October 25, 2009
During this public comment period, the CMS received a total of seven public comments. Five of these individuals commented during the first comment period, but made more extensive remarks during the second comment period. One comment came from a health care provider and developer of pulsatile insulin in the U.S., a second came from a laboratory and business affiliate of the developer, a third came from a technology investor, a fourth came from a clinical investigator-author, the fifth came from an officer in a diabetes organization, and the sixth and seventh came from unaffiliated persons. Five favored immediate coverage, one explained his study and favored further research, and the last commented on health care reform.
No new clinical studies were provided. Several tangential references without complete citations were recommended for review. Several commenters discussed judicial decisions regarding coverage in California.
We note that CMS was not a party to the State court decisions cited by commenters, nor is the Agency bound by those decisions. We also note that such materials do not constitute scientific evidence upon which we rely when making national coverage determinations.
Because of the length of the commentary by several of the commenters, the comments have been tabulated by topic below. Full text comments without personal health information may be viewed at: www.cms.gov/mcd/viewpubliccomments.asp?nca_id=231.
| Commenter |
Comments |
CMS Response |
| |
Scope of OIVIT |
|
Aoki *Author *Inventor & patent holder of treatment algorithms *Consultant to Aoki Diabetes Research Institute (ADRI) * 1st period comments too * Favored coverage |
*Raised objections to including insulin infusion regimens other than the patented Aoki MAT treatment in the NCD review and analysis. |
*Multiple variations of intravenous insulin infused in the outpatient setting were considered and included under the CMS designated class name OIVIT.
*CMS did review the patents and patent applications for MAT-type therapy and MAT-type infusion pumps to better determine the scope of the proprietary treatment(s).
*In its review, CMS did distinguish between pulsatile and continuous infusion, the characteristics of the pulses, and other ancillary aspects of treatment. |
Arcangeli *Co-author *Lab research associate *Business associate in Metabolic Industries, Inc. * 1st period comments too *Favored coverage |
*Stated that the MAT is not a broad class of therapies, but is a single therapy with multiple names. |
*Multiple variations of intravenous insulin infused in the outpatient setting were included under the CMS designated class name OIVIT. The therapies were reviewed as a class and as discrete entities. |
Parsons *International Technology Ventures *1st period comments too *Favored Coverage |
*Stated that it was a gross simplification to consider MAT within OIVIT. |
*See above response. |
Aoki |
*Stated that he did not use glucose clamps in MAT and that discussion of studies that employed insulin infusion as part of a glucose clamp are not germane.
|
*Animal and human physiologic and short-term clinical studies of insulin infusion, especially those of pulsed insulin infusion were reviewed for completeness. Specific animal studies were recommended by Goodner and Polonsky. *The glycemic clamp technique provides a relatively high degree of precision about the insulin infusion and the subsequent glucose utilization by target tissues and any glucose production by the liver. |
Arcangeli |
*Stated that a euglycemic clamp study does not resemble MAT therapy. |
*See above response.
*The clamp technique is used to maintain a relatively constant glucose level. This would be important when comparing the efficacy of various insulin infusions (including dose, continuous vs pulsatile, and pulse characteristics). The Biostator was one of the devices used for clamps. This closed-loop system also could be used for insulin infusions in clinical and research settings where the precision of the glucose level could vary more, e.g, where the goal is hypoglycemia avoidance.
*In a "Restoration of Glucose Homeostasis in Insulin-dependent Diabetic Subjects: An Inducible Process", Foss, Vlachokosta, Cunningham, and Aoki in 1982 state "The artificial ß-cell unit (Life Science Unit, Miles Laboratory, Inc, Elkhart, Indiana)" "(known as the Biostator and no longer commercially available)" " used in this investigation is composed of a blood withdrawal system coupled to a fixed matrix glucose oxidase-glucose sensor that continuously monitors the blood glucose level. On the basis of this glucose level or the rate of change of the blood glucose concentration, the infusion system delivers insulin or glucose into a peripheral vein to achieve a pre-determined glucose level. This device is capable of returning relatively uncontrolled insulin-dependent patients to the euglycemic state in 2-3 hours and maintaining pre- and postprandial blood glucose levels in the normal range (70-150 mg/dl) for as long as seven days." Of note, the insulin infusions in this study were not noted to be pulsatile.
*References for clamps are included in the CMS bibliography. Of particular note is the reference "Development of the Biostator Glucose Clamping Algorithm" by Clemens et al. 1982. "Infusion is activated to maintain a patient’s blood glucose concentration within a selected range." |
Arcangeli |
*Stated that the animal studies are not germane. |
*Animal and human physiologic and short-term clinical studies of insulin infusion, especially those of pulsed insulin infusion were reviewed for completeness. As stated in the NCA review, the results were contradictory. Animal studies may provide important guidance for subsequent human clinical studies. See comments by Goodner and Polonsky. |
Parsons |
*Stated that the animal studies are not germane. |
*See above response. |
Aoki |
*Stated that only ADRI and Metabolic Industries have licenses for his therapy and that other entities have been ordered to "cease and desist".
Stated that CMS identified non-licensed entities as licensees. |
*CMS delineated a variety of groups that offered this class of therapy. CMS noted that franchise-type arrangements exist. CMS does not verify licensing agreements. The information in the cited parentheses reflects reference documents in the bibliography. |
Arcangeli |
*Stated that MAT is a patented treatment-available only at licensed centers. Other entities have been ordered to "cease and desist", and the SEC has been notified. NCA referenced websites have disappeared. |
*CMS has retained hardcopies of referenced website material. |
Parsons |
*Stated that only ADRI and Metabolic Industries have licenses for this therapy |
*See response to Aoki comments above. |
| |
Disease Background |
|
Parsons |
*Stated that 445,000 are disabled by diabetes and that MAT would prevent problems and save money. |
*No further references were provided. |
Scott *Diabetes organization not found with web search *1st period comments too *Favored coverage |
*Stated that diabetes is a chronic disease which affects millions of patients via a myriad of physical systems and that MAT would prevent problems and save money.
|
*No further references were provided. |
| |
Treatment Background |
|
Arcangeli |
*Stated that RQ measurements are used to determine insulin doses, but that no computer inputs or algorithms are used. *Later stated that "CIIIT uses a complicated therapy algorithm involving high doses of insulin and should never be casually imitated. Providers and staff must complete a training course…." |
*These discrepancies in the description of MAT therapy have not been reconciled. The patent descriptions provide no further illumination. *See comments by Goodner, Heinemann, and Millis. |
Arcangeli |
*Stated that the inclusion of papers on the various routes of insulin administration may have impaired the analysis of OIVIT. |
*Information on the various routes of insulin administration was included in the background section because insulin is typically self-administered except in the hospital setting. |
Arcangeli |
*Stated that MAT is not an alternative therapy; it is an adjunctive therapy for refractory or problematic patients. *Stated that the pharmacologic effects of MAT differ from those of SQ insulin. |
*CMS understands that the MAT subset of OIVIT is provided as adjuvant therapy and is dosed episodically. *CMS understands the differences in the pharmacokinetic/ pharma-codynamic profiles of insulin given by different routes. * The NCD non-covers all out-patient intravenous insulin administration. |
| |
FDA |
|
Aoki |
*Stated that other companies have made questionable claims including FDA approval of therapy. |
*The FDA has not approved or cleared OIVIT treatment regimen(s) or specific devices for OIVIT treatment regimen(s). |
| |
Nature of therapy |
|
Aoki |
*Stated that MAT therapy is not directed at glycemic control, but rather is directed at the provision of insulin to target tissues to improve metabolism and thus reduce complications. |
*Regardless of the stated etiology of an intervention, blinded, randomized, outcome studies with hard clinical endpoints are necessary to establish efficacy. One of the benefits of MAT therapy listed on the website www.adri.org/faqs.html#1 , however, is "improved blood glucose control’. *See comments by Lachin. |
Arcangeli |
*Quoted the NCA on the limitations of glycemic control in the prevention of cardiovascular disease and macrovascular or hypertensive renal disease and the reversal of microvascular complications. |
*These limitations were well delineated in the CMS MEDCAC in the Summer of 2006. Investigators were encouraged to consider other etiologic pathways.
*Regardless of the stated etiology of an intervention, blinded, randomized, outcome studies with hard clinical endpoints are necessary to establish efficacy.
* See comments by Goodner, Heinemann, Lachin, and Polonsky. |
Arcangeli |
*Stated that genetic over-expression of hepatic enzymes in glucose control (hexokinase IV, phosphofructokinase, and pyruvate kinase) was not germane.
*Stated that the Torres 2009 reference is incorrect. |
*This enzymatic pathway is the one addressed in the "Sleeping Liver" by Aoki. Investigators have explored induction of these pathways by pharmaceutical agents and gene expression. The results have not been uniformly successful, e.g., sometimes very high levels of over-expression are required. This pathway may have significance, but solid clinical data delineating the means for safely and effectively activating this pathway have yet to be demonstrated. Indeed Felber et al. in a series of articles in the 1970s and 1980s in which RQs are employed postulate that glycogen storage may be more important than oxidation in some forms of diabetes and obesity.
*The correct citation was O’Doherty 1999 and not Torres 2009. |
Parsons |
*Stated that large bolus doses of insulin mimic the action of the normal pancreas and enhance the effect on tissues and that Dr. Aoki had invented a way to achieve this without simultaneously inducing hypoglycemia. *Stated that MAT improved glycemic control, but also appeared to have effects on diabetic complications which were not related to glycemic control. Stated that this was the reason that CMS rejected the results of the Dailey study. *Stated that clinical outcomes are more important than etiology. |
*Regardless of the stated etiology of an intervention, blinded, randomized, outcome studies with hard clinical endpoints are necessary to establish efficacy. An estimated annualized rate of glomerular function decline is not a hard clinical endpoint. In addition, the primary endpoint did not reach statistical significance after 12 months. Drop-out was high prior to 12 months and prior to the post hoc observation interval of 18 months. This limits generalizability of any results. Furthermore, there was no adjustment for sequential statistical testing. Any statistical significance would be diminished. See comments by Hayward and Lachin. *Did not reconcile the results between the Aoki 1999 paper in which decreased renal function decline was attributed, in part, to improved glycemic control and the Dailey paper in which glycemic control was non-contributory. |
| |
Metabolic assessments |
|
Aoki |
*Discussed how RQ measurements assess hepatic glucose oxidation. *Reported that infection or pain could alter RQ response to MAT treatment. |
*No human clinical data to support claims were provided. *The RQ data from Foss, Vlachokosta, Cunningham, and Aoki "Restoration of Glucose Homeostasis in Insulin-dependent Diabetic Subjects: An Inducible Process" (1982) were obtained from 5 normal and 5 type 1 diabetic subjects using a Biostator and continuous insulin infusions. *Felber et al. in a series of publications in the 1970s and 1980s in which RQs were employed observed different degrees of carbohydrate oxidation in type 1 and type 2 diabetes patients. *See comments by Goodner, Heinemann, and Millis. |
Arcangeli |
*Stated that RQ measurements are used to determine insulin doses, but that no computer inputs or algorithms are used. |
*No further treatment information was provided. |
Parsons |
*Stated that RQ levels assess therapeutic efficacy and have been essential for obtaining clinical data for published papers. |
*No further validation data were provided. |
Aoki |
*Stated that inclusion of urinary nitrogen measurements is not required. Cited early physiologic work by Foss. (Specific citation data not provided.)
|
*RQ measurements are gross assessments of metabolism. They are most reliable when conducted in a steady-state condition, e.g., the fasted state and immobile state, and when all components of nutrition are included. No subsequent urinary nitrogen data from clinical trials was provided. See comments by Goodner, Heinemann, and Millis. See editorial by Heinemann 2001. |
Arcangeli |
*Stated that urea nitrogen measurements are not usually obtained. |
*CMS presumes that this refers to urinary urea nitrogen levels, which may be obtained as part of comprehensive RQ assessments * No further references were provided. |
Aoki |
*Stated that although potassium levels may decrease with insulin infusions, this phenomenon occurs only with longer infusions. ADRI does monitor potassium levels in patients with borderline low levels. |
*No human clinical data to support claims were provided. |
Arcangeli |
*Stated that potassium is not routinely monitored in MAT. |
*See insulin drug labels for IV administration. |
Aoki |
*Stated that hypoglycemia (< 50 mg/dl) occurred in < 1% of ADRI treatment sessions. |
*Hypoglycemia is a well established complication of IV insulin even in a highly monitored in-patient setting. The commenter did not provide information that reconciled expected hypoglycemia rates and reported clinic rates. Monitoring procedures and glucose rescue rates were not provided. |
Parsons |
*Stated that hypoglycemia (<50 mg/dl) occurred in < 1% of ADRI treatment sessions (unpublished data). Encouraged CMS to visit ADRI. |
*See above response. |
Arcangeli |
*Stated that there have been no reported cases of hypertriglyceridemia or pathologic lipid deposition with MAT. |
*Concerns about hypertriglyceridemia and abnormal lipid deposition have been raised for pharmaceutical agents which activate enzyme pathways for hepatic glucose. These putative adverse events are targets for rigorous safety monitoring. Because these pathways are the same as/overlap with those activated by MAT, systematic collection of such data in OIVIT is warranted. *No human clinical data to support claims were provided. |
Arcangeli |
*Stated that adverse events did not occur because the procedure mimics nature and monitoring was done. |
*No information on monitoring procedures was provided. |
| |
Evidence Base |
|
Aoki |
*Stated that "…where no effective therapy is available, new therapies that show improvement, even in a small number of patients, are usually called ‘promising’ rather than ‘no evidence of benefit’. |
*The metabolic pathway this therapeutic modality purports to activate may play a role in diabetes/diabetes complication management. Indeed, pharmaceutical agents that alter these enzyme pathways for hepatic glucose are under development. The data generated thus far as well as any that might be generated by the ADTC studies, however, while sufficient for hypothesis generation, are not/will not be definitive . *See comments by Hayward, Heinemann, Lachin and Polonsky. See ClinicalTrails.gov. See ADTC research Compendia. |
Arcangeli |
*Stated that the current evidence base was sufficient to support coverage of CIIIT brittle diabetes, hypoglycemic unawareness, excess hypoglycemic with intensive therapy, refractory hypertension, refractory orthostatic hypotension, and diabetic nephropathy. *Stated that they would be willing to present observational data for other indications. |
*CMS uses the principle of evidence-based medicine in its decisions. These principles are outlined in the methods section and the accompanying appendix.
*See Mulrow 2001. See Duke University Medical Center Library "Evidence-based Clinical Practice Resources" at the following website www.mclibrary.duke.edu/subject/ebm/ebmpyramid.html. |
Arcangeli |
*Repeatedly referred to the MPCQT reviewer in its comments. Challenged the 2001 Blue Shield technology assessment and stated that the incidence of diabetic renal failure would not be so high if the alternatives delineated in the technology assessment were effective. *Stated that Blue Shield had a conflict of interest because of the financial implications of the court rulings. |
*The acronym MPCQT referred to the Blue Shield Medical Policy Committee on Quality and Technology. The comments appear to have been taken from ADRI commentary on the 2001 Blue Shield technology assessment. *CMS conducted an independent review of an updated database. CMS includes descriptions of all available technology assessment in its NCD document. |
Arcangeli |
*Commented that the Hayes technology assessment concluded that the treatment had potential, but unproven benefit (vs the lowest rating no proven benefit and/or not safe). For this reason, CMS should provide coverage and help design future studies. |
*CMS conducted an independent review of an updated database. CMS included descriptions of all available technology assessment in its NCDs. The Hayes group was contacted directly because it is a proprietary assessment group. The letter ratings are not for public release. |
Arcangeli |
*Stated that the Aoki 1993 study in 20 "brittle" patients constituted a controlled study because the degree of glycemic control improvement was similar to or superior to that in the DCCT with one-third of the expected hypoglycemic events, |
*The Aoki 1993 paper described a small case series with an extrapolated historical control, not a separate contemporaneous population. Variables which could have contributed to the claimed improvement in hypoglycemic awareness/decreased hypoglycemic event rate, e.g., increased patient support vs the pulsatile insulin, were not delineated. |
Arcangeli |
*Stated that the Aoki 1995 study in 3 patients with refractory postural hypotension constituted a controlled prospective study. *Stated that these results had been replicated in a larger patient group. |
*The Aoki 1995 paper described a case series with a pre-post sequential self-control design. *No publication for the replicate study was provided |
Arcangeli |
*Stated that the Aoki 1995 study of circadian blood pressure changes is significant because it showed improved glucose control and biological change which will result in decreased end-organ damage. *Noted that CMS would have like to have known about hypoglycemic events, and stated that "This is both the joy and grief of researching CIIIT-we cannot afford to measure and report the entire list of parameters that CIIIT affects in other systems…". |
*The Aoki 1995 paper described a post hoc analysis of a patient population not otherwise described in the published literature. The ambulatory blood pressure readings may be significant, but describe a surrogate endpoint. The relationship between glucose control, which improved by 0.5 HbA1c unit % and blood pressure control was not assessed. Given the potential relationship between renal dysfunction and blood pressure and the disparate relationship between glycemic control and renal function decline in the Aoki 1999 and Dailey papers, it would have been important to conduct such analyses. Given that altered circadian blood pressure and hypoglycemia, especially hypoglycemic awareness, may be reflections of autonomic neuropathy, having such data from the apparently largest MAT study would have been useful for assessing risk:benefit albeit limited to persons with Type 1 diabetes. |
Arcangeli |
*Discussed the Aoki 1995 cross-over study in patients with Type 1 diabetes. *Stated that there was no significant drop-out. *Stated that the drop-out rate during the run-in has no impact on interpretation of the data. *Stated that glycemic control was not contribu-tory to the lowered anti-hypertensive medication requirements in this. *Stated that CMS discussion of proteinuria was irrelevant. |
*Limited conclusions can be drawn from small studies with large drop-out-whether within the run-in period or the actual treatment period. Nine subjects of 41 dropped out during the run-in period and another 6 were excluded from the treatment period analyses. Furthermore, study design depends on maintaing blood pressure at steady state. There were small increases in systolic blood pressure. *Proteinuria was not discussed for this study by CMS. |
Arcangeli |
*Stated that CMS erroneously focused on the lack of changes in proteinuria and any effects from increased monitoring and treatment feedback in this long-term nephropathy case series study by Aoki 1999. |
*Limited conclusions can be drawn from uncontrolled case series data with variable observation periods.
*Glycemic control was reportedly improved by 1.0 HbA1c unit % in these patients and renal function decline blunted. No correlative analyses for these two outcomes were conducted. *CMS stated that "Although mean glycemic control improved"…, " mean urinary protein and creatinine clearance did not change". In the absence of a control population and variable observation periods, *CMS does not concur that the lack of change in these parameters clearly demonstrated that renal function decline was blunted in response to OIVIT. *The commenter did not reconcile the disparate etiology between the Aoki 1999 paper in which decreased renal function decline in an uncontrolled patient population was attributed, in part, to improved glycemic control and the Dailey paper in which glycemic control was non-contributory.
|
Arcangeli |
*Discussed the Dailey paper. *Stated that there was no significant drop-out and that the primary reason for drop-out at the time of study extension was "the time required for weekly clinic visits". Indicated that Dailey drop-out rate was not unexpected and cited Lewis 1993 3 year study (26%) and Mogensen 1995 2 year study (26%).
*Stated that because an intent-to-treat analysis was not performed, any discussion of such an analytic deficiency by CMS because the results might be positive.
*Stated that CMS wrote that "the absence of reduction in urinary protein was unexpected". *Stated that CMS discussion of anti-hypertensive therapy and dietary protein restriction was superfluous. *Stated that any discussion of the DCCT was not-relevant because patients with overt nephropathy, such as those in the Dailey study, were excluded from the DCCT. |
*The primary endpoint was annualized creatinine clearance at 12 months. Ninety subjects enrolled; 71 completed 12 month; 49 completed 18 months. The drop-out rate, the failure to correct for multiple observations, the use of a surrogate endpoint, and the study of Type 1 diabetic patients limits assessment of efficacy and generalizability to the Medicare population. See comments by Hayward and Lachin. *CMS could not locate the statement attributed to it in the proposed document "..the absence of a reduction in urinary protein in both groups was also unexpected". *CMS discussed the DCCT in the context of the available knowledge base. |
Arcangeli |
*Stated that CMS "also speculates that it would take years of CIIIT to prevent progression of end-stage renal disease" while also having stated that the current Dailey and Aoki studies suggest that" CIIT can stave off renal failure for years". |
*CMS noted the need for long-term clinical trials with hard endpoints and the limitations of surrogate endpoints. * See comments by Hayward, Lachin, and Polonsky. |
Parsons |
*Discussed both the Aoki 1999 and Dailey papers. |
*See above response sections. |
Parsons |
*Stated that "…CMS has steadfastly refused to consider anecdotal evidence of long term use of the technology in 38 patients over an average of more than 13 years….". Stated that CMS did not consider the long-term results on these 38 patients which were presented to the Agency at a meeting because they were not peer-reviewed and published. *Stated that CMS does not give credence to testimonials in public comments whereas the judge in the CalPERS case did. |
*CMS uses the principle of evidence-based medicine in its decisions. These principles are outlined in the methods section and the accompanying appendix. See Mulrow 2001. See Duke University Medical Center Library "Evidence-based Clinical Practice Resources" at the following website www.mclibrary.duke.edu/subject/ebm/ebmpyramid.html.
|
Parsons |
*Stated that there are areas where Dr. Aoki and CMS could co-operate to develop conclusive trials. *Stated that this "is precisely the kind of technology envisioned when the Medical Clinical Trials program was established by Congress". *Stated that effectiveness could be shown via (a) demonstration project(s) in Medicare and/or Indian Health populations. |
*The Coverage with Evidence Development program may provide coverage for items/devices/services under select criteria at the discretion of CMS. *There is no statutory Medical Clinical Trials program. President Clinton issued an executive order to encourage enrollment of Medicare beneficiaries in clinical trials. Routine costs may be covered for appropriate deemed clinical trials. *CMS (ORDI) may also conduct/arrange for demonstration projects regarding coverage based on statutory decree and funding or internal decision. *See comments by Lachin, and Puklin. *See section for Professional Societies regarding the IHS. |
Parsons |
*Stated that, in the setting of underpowered studies, CMS has not established that the treatment does not work and so should provide coverage. |
*The evidence presented is insufficient to conclude that OIVIT is, as set in statute, "reasonable and necessary" for any treatment. |
Parsons |
*Stated that treatment should be provided on a compassionate use basis. |
*CMS uses the principle of evidence-based medicine in its decisions. |
Scott |
*Stated that many reputable organizations had published studies and would not have done so if there were reservations. *Stated that there was no evidence that MAT did not improve health outcomes. *Stated that double-blinding would be unethical. |
*No additional published studies were provided. *See comments by Heinemann, Lachin, and Polonsky. |
Scott |
*Stated that patient/provider testimonials were significant evidence and were not given sufficient consideration by CMS. |
*CMS uses the principle of evidence-based medicine in its decisions. *See prior CMS comments. |
Weinrauch *Author *1st period comments too *Favors more research |
*Discussed the Dailey and derivative Weinrauch studies. *Discussed an abstract presented at the ADA 2009. *Stated that additional studies in select populations is warranted, but that feasibility and funding issues resulted in discontinuation of the technique at his institution (Harvard-Joslin) in the 1990s and that determination of benefit with hard endpoints for diabetic complications is prohibitively expensive for the private sector. *Stated that additional studies, without blinding, are warranted. |
*The 2009 ADA abstract was not provided. *Prospective, randomized, blinded controlled studies provide the best data-especially when compliance or efficacy might be affected by differential provider input/support. *See comments by Heinemann, Lachin, and Polonsky. |
| |
Experts |
|
Aoki |
*Stated that experts in OIVIT therapy should be consulted and that the field is small because funding and research has been directed at glycemic control.
|
CMS reasonably accords greater evidentiary weight to independent expert opinion from persons who do not have personal interests in the matter under consideration. In the proposed decision memorandum we stated that we expected "to received expert opinion on the proposed decision". We would reasonably expect to receive such opinion after the publication of the proposed decision itself. CMS makes its coverage decisions on the basis of available scientific data. More evidentiary weight is given to more rigorous data. |
Parsons |
*Stated that CMS had not yet obtained expert opinions on OIVIT ,and MAT prior to publishing the proposed decisions, but would be doing so. |
Parsons |
* "Without such firsthand visits" [with patients and doctors] "CMS will continue to ‘hide behind’ the pontification of ‘experts in the field’ with no actual experience using the technology….". |
| |
Other |
|
Aoki |
*Quoted the California state administrative law judge "The Blue Shield of California’s Medical Policy on Quality and Technology is not enforceable in this action. The policy was not enacted until February 2001, almost three years after the denial actions that compose this action,….." . |
*The comments do not challenge the Blue Shield technology assessment. Rather they appear to challenge the use of a negative technology review and assessment to deny insurance claims for MAT. A formal Blue Shield policy on MAT was developed after some claims had been paid. |
Aoki |
*Stated that CMS should review the CalPERS decision about coverage. |
*CMS previously reviewed the CalPERS decision and has included it in the NCD bibliography. CMS bases its decision on its review of the scientific literature.
|
Arcangeli |
*Stated that the critique of the Blue Shield technology assessment has been rebutted (in court) and CMS has failed to address this in the proposed NCA document. Stated that he would repost. |
*CMS previously reviewed the Blue Shield technology assessment, the CalPERS decision, and the Blue Shield decision. All are included it in the NCD bibliography. CMS bases its decision on its review of the scientific literature. |
Parsons |
*Stated that "outstanding results" " swayed the courts of California to conclude that Metabolic Activation Therapy is both safe and effective". Stated that "CMS has made no mention of these court decisions even though evidence of the proceedings was presented to CMS." *Stated that the CMS decision further increased the dichotomy between the court mandated coverage in California and the lack of coverage by insurance carriers. |
*See above response. |
Scott |
*Stated that Blue Shield was sued over coverage and ordered to pay for coverage. *Stated that the fiduciary agents for CalPERS were sued over coverage, and that an administrative law judge determined that MAT was not an investigational therapy. *Stated that CMS should have considered the court cases in the NCD. |
* See above response. |
Parsons |
Stated that CMS stated in a recent meeting that potential savings to Medicare are not of interest to CMS. |
*The comment appears to be based on a misunderstanding of the statement that is routinely delivered to those developers of drugs, devices, and services who make presentations to the Coverage and Analysis Division (CAG) in CMS. CAG prepares NCDs on the basis of scientific data to determine that coverage which is "reasonable and necessary" for Medicare beneficiaries. It does not perform cost analyses. It does not prepare comparative effectiveness studies. It does not prepare cost effectiveness studies. It does not establish reimbursement levels. |
Parsons |
*Discussed the start-up costs for clinics. Believes that operating costs will come down in an analogues fashion to hemodialysis clinics. Noted that no formal cost-benefit analyses have been conducted. |
*CMS makes its coverage decisions on the basis of available scientific data. |
Unspecified *Healthcare reform issues |
*Expressed concern about health care reform and loss of care for senior citizens |
*CMS makes its coverage decisions on the basis of available scientific data. |
| |
Testimonial |
|
Arcangeli |
*Started that patients in the studies experienced improved physical outcomes and quality of life and that this is why they continued treatment at an off-campus site. |
*No additional published studies were provided. |
Patient * Favored coverage |
*Reported improvement in glycemic control and neuropathy |
*No additional published studies were provided. |
VIII. Analysis
Introduction
National coverage determinations (NCDs) are determinations by the Secretary with respect to whether or not a particular item or service is covered nationally under title XVIII of the Social Security Act. §1869(f)(1)(B). In order to be covered by Medicare, an item or service must fall within one or more benefit categories contained within Part A or Part B, and must not be otherwise excluded from coverage. Moreover, with limited exceptions, the expenses incurred for items or services must be "reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member." See §1862(a)(1)(A) of the Act.
The Medicare regulations at 42 CFR 410.32(a) state in part, "…diagnostic tests must be ordered by the physician who is treating the beneficiary, that is, the physician who furnishes a consultation or treats a beneficiary for a specific medical problem and who uses the results in the management of the beneficiary’s specific medical problem." Thus we look for evidence demonstrating how the treating physician uses the result of a test, such as the respiratory quotient (RQ), for the management of a patient with diabetes who is undergoing intravenous insulin treatment and whether insulin treatment strategies that incorporate diagnostic respiratory quotient testing to guide insulin therapy improve health outcomes compared to strategies without RQ testing. This section presents the agency’s evaluation of the evidence considered and conclusions reached for the assessment.
This section presents the agency’s evaluation of the evidence considered and the conclusions which were reached.
Discussion
Outpatient intravenous insulin therapy (OIVIT) encompasses a large array of treatment regimens with associated services and testing. The various individual components of OIVIT, e.g., insulin, insulin infusion, pump use, metabolic cart measurements, and laboratory testing, may have medical uses in conventional treatment regimens for diabetes and other conditions. We described many of the more conventional uses of these components in the background sections. We described the use of self-administered subcutaneous shots of insulin for daily outpatient diabetes management. We described the inpatient use intravenous insulin for patients with serious or life-threatening conditions, such as diabetic ketoacidosis or diabetic hyperosmolar coma, in the absence of RQ assessments to guide dosing. We described the special requirements for intravenous insulin including insulin dilution, concomitant potassium administration, monitoring of glucose, electrolyte, and fluid levels, and skilled nursing. We described the use of inspired and expired gas measurements in the inpatient setting on a sporadic basis for the adjustment of ventilation and parenteral nutrition parameters. We described the occasional use of respiratory gases in the outpatient setting for the determination of basal metabolic rates and in cardiopulmonary stress testing. These conventional uses were contrasted with the less conventional uses of these components in OIVIT. The differences in their frequency of use were also compared. In this decision, we are not making (a) coverage determination(s) regarding these more conventional uses. Coverage for such conventional uses may be determined by other local or national Medicare determinations and will not be considered here. The most commercially available OIVIT procedure in the U.S. involves serial infusions of insulin in a pulsed fashion. In contrast to the inpatient use of intravenous insulin administration, the therapy is adjunctive to the patient’s usual diabetes management regimen. Metabolic cart measurements of inspired and expired gases, specifically respiratory quotients, (RQ), may be used for the putative assessment of the oxidative response to insulin dosing and for the determination of subsequent insulin dosing. In contrast to their uses in cardiopulmonary testing and Intensive Care Unit (ICU) management, multiple respiratory gas measurements for serial RQ assessments are often obtained on a single day. Specific criteria describing how such assessments determine subsequent insulin infusion dosing are not available in the methods sections of the published literature or the patents for OIVIT. (Aoki 1993, 1995a, 1995b, 1995c, 1999, Dailey 2000, Weinrauch 2007, 2009 a, 2009b, Aoki patent series, Gilbert patent series) The other parameters used to determine subsequent insulin dosing (possibly patient characteristics, treatment response history, and/or diagnostic test results) are not delineated in the methods sections of the published literature or the patents. Protocol measures to enhance safety during intravenous insulin administration (except for the use of ad libitum carbohydrates) and to systematically monitor for adverse events are not available in the methods sections of the published literature or the patents.
CMS searched for evidence regarding OIVIT, including evidence pertaining to the use of its components in the way they are utilized in OIVIT regimens. Although we focused on clinical trials, we did consider animal studies (See Background) and short-term/human physiology studies (See Evidence) because these types of studies are frequently used to establish primary efficacy, identify treatment protocol variables that impact efficacy, and identify patient or disease indication variables that impact efficacy prior to more extended clinical trials in human subjects.
Most of the animal and short-term/human physiology studies differed from therapeutic clinical studies in that the insulin dose was replacement dosing and not intermittent adjunctive dosing. Nonetheless, as in the three cited animal studies, the efficacy results in the short-term/human physiology studies were contradictory. Some studies demonstrated improved glycemic control or nutrient utilization whereas others did not. Such inconsistency suggests the presence of important variables in the regimen, technique, patient characteristics, or disease state which require identification. Thus far investigators have not been able to determine which variables, whether intrinsic or experimental, except perhaps pulse rate, glucagon level and duration of infusion, contribute to these disparate results. (Porksen 1998, Zarkovic 1999, 2000) Of note, respiratory quotients were seldom included in short-term/human physiology study protocols. As such, their role in future therapeutic clinical trial regimens was not clearly defined. (Meistas 1985)
CMS did identify nine published manuscripts for five studies in which outpatient IV insulin therapy was employed. The same proprietary treatment regimen was utilized. Only patients with diabetes were studied. Patient variables, (e.g., age, diabetes type, disease severity, and co-morbid conditions), or treatment variables, (e.g., pulse parameters, respiratory quotient response, and duration of therapy), which could impact therapeutic utility were not adequately addressed in the studies. None of the studies served as registration trials for FDA devices or drugs. No IND or IDE information was included in the methods sections.
All of these longer-term studies have significant limitations in their design, study size, completion rate, type of patient enrolled, underlying hypothesis, robustness of their endpoints, and prospective safety monitoring. Indeed, none of the studies were blinded. Only two were randomized. Most were case series. The design issues make it difficult to determine whether the investigational treatment actually results in health benefits and whether any apparent benefit is due to increases patient supervision and support, increased insulinization (which could also be achievable by more aggressive standard therapy), or the putative unique aspects of the OIVIT intervention. The design issues also limited the ability to identify and assess potentially important patient variable for efficacy and safety.
All of the studies were small (N < 100). Indeed, four manuscripts were based on populations that were subsets of larger study populations and not independent, replicate studies. Therefore, despite the number of studies, the total patient exposure in the studies was less than 250 patients. Studies of sufficient duration to establish efficacy and/or durability were: a) too small at initiation or through attrition or b) had design shortcomings. For example, analysis of only those patients who completed the studies instead of all enrollees in a standard intent-to-treat analysis can introduce selection bias. In addition, large differences in the clinical and demographic findings between the screened patient population and the entrant population limits generalizability. The small total study population size also limits assessment of benefit in patient subgroups including those from different socioeconomic strata, different ethnic/racial groups, with different diabetes types, with different degrees of disease severity and with different types of co-morbid disease.
In fact, only Type I diabetic patients were studied, limiting the generalizability of any results for OIVIT to the Medicare patient population with its preponderance of Type 2 patients. Similarly, no non-diabetic or perioperative islet cell transplant patients were studied, precluding generalizability of any evidence to putative indications in those patient populations. In addition Felber, et al. observed different carbohydrate oxidation patterns in response to a standardized glucose challenge in Type 1 versus. Type 2 diabetic patients. (Felber 1977, 1981a, 1981b, Golay 2002, Meyer 1980) This suggests that the respiratory quotient (RQ) may not have the same utility as a surrogate endpoint or for diagnostic use in guiding insulin dosage in all patient populations even if OIVIT were to be efficacious. Many of the endpoints for efficacy were subjective or poorly defined a priori. None were hard clinical endpoints or non-surrogate endpoints. Several studies had multiple endpoints and only reported on the variables that were statistically significant in those patients who had completed the study. The investigators did not make statistical corrections for multiple comparisons (use of multiple endpoints) and did not conduct intent-to-treat analyses on the entire study population. The researchers for one study extended the length of the study until there was statistical significance.(Dailey 2000) In another study of the effects of OIVIT on the cardiovascular factors affecting renal dysfunction, the scientists conducted the wrong statistical comparisons and also did not address baseline imbalance for mean arterial pressure (MAP), and perhaps glycemic control, that favored the OIVIT intervention arm. (Weinrauch 2007) One study used a self-report survey tool developed by the progenitor of the pulsatile insulin regimen and not otherwise used or validated by diabetes researchers. (Hammond 2004, Weinrauch 2009) The investigators further modified this survey instrument by excluding questions in which there was no discordance between the investigational and control treatment cohorts, which were the majority of the questions in the survey. Such selective alteration increases the likelihood of a obtaining a positive, but factitious statistical difference between two treatment populations,
No pre-specified protocols for the assessment of safety endpoints (e.g., hypoglycemia, hypokalemia, serum lipids, and tissue lipids) with diagnostic tests were delineated in the studies despite the fact that intravenous insulin in known to have a narrow therapeutic index (safety margin) and that dyslipidemia and organ/peri-organ lipid deposition are putative risks with activation of the glucokinase pathway. There was no rigorous reporting of adverse events in the study manuscripts. The discordance between the well established risk for hypoglycemia and hypokalemia with intravenous insulin even in the highly monitored inpatient setting and the reported near absence of risk despite repeated exposures to an invasive procedure with OIVIT has not been explained by the investigators. We have no data on the total insulin dosing per session, serial glucose levels, carbohydrate consumption per session, or weight changes from ad libitum carbohydrate ingestion, all factors which could help explain this paradox.
There are also unanswered questions about the underlying therapeutic principles for OIVIT. The earliest reports claimed improvements in glycemic control. Putative improvements in the rate of renal function decline, antihypertensive medication reduction, and perceived disability in later studies, however, were not related to the glycemic control achieved with OIVIT. Indeed the data suggested that the mean glycemic control was equivalent or better in the control group. (Weinrauch 2007) Correlative analyses between hemoglobin A1c measurements of glycemic control or changes in glycemic control and rate of renal function decline, antihypertensive medication reduction, or perceived disability were not conducted. No rationale for this epiphenomenon of positive results in the absence of improved glycemic control was provided. It may reflect the absence of a true treatment effect and the finding of spurious positive results in the setting of statistical analyses which are uncorrected for the effects of multiple endpoints.
The function of the diagnostic tests potentially associated with OIVIT (e.g., blood/serum glucose levels, serum potassium levels, respiratory gas measurement, and urine urea nitrogen levels) has not been well delineated and validated in the published trials and patent material for OIVIT. (Aoki 1993, 1995a, 1995b, 1995c, 1999, Dailey 2000, Weinrauch 2007, 2009a, 2009b, Aoki patent series, Gilbert patent series)
Professional grade chemistry testing for potassium and glucose for the monitoring of hypokalemia and hypoglycemia respectively in OIVIT is an important element of safety. The use of home or non-professional glucose monitors is not adequate. (Diabetes Today 2009, FDA-Gaines Meter Error, FDA Glucose Meter Alerts, FDA Glucose Meter Advisory Committee Meeting 2001, FDA Meter Review Criteria, Hovorka 2006, Kost 1998, Meguro 2005, Perera 2009) The failure to have a monitoring algorithm for hypokalemia and hypoglycemia in OIVIT is problematic because of the known clinical risks.
Algorithm information for use of the respiratory quotient was absent from the methods sections of all papers. Furthermore, no head-to-head comparative studies employing OIVIT with and without RQ to establish the validity of RQ testing have been conducted. None of the published trials were structured to assess the role of the respiratory quotient either as a marker for efficacy (if any) or as a tool for any subsequent insulin dosing. Response rates, as indicated by an achievement of predetermined respiratory quotient thresholds or changes in respiratory quotient values were not presented. Correlation of respiratory quotient (RQ) values or the magnitude of change in respiratory quotient values with clinical outcomes was not presented. No studies assessed the impact of scheduled or ad libitum carbohydrate ingestion or antecedent meal content on respiratory quotient measurements. (Austin 2008, Benade 1973, Blundell 2002, Maffeis 2004, McGregor 1995, Root 1944, Talbott 1938) No studies included urine collections as part of their respiratory quotient determinations. None of the studies were structured to assess whether the urinary component (UUN) of RQ testing contributed to efficacy of any kind. No studies addressed the absence of steady state conditions in the presence of glycosuria (glucose loss through the urine) and albuminuria (protein loss through the urine). No studies addressed factors that could affect the reliability and predictive value of the respiratory quotients such as technique requirements, device variability and standardization issues.
CMS did not identify any high quality clinical trials for OIVIT for any clinical indication. None of the studies clearly established any efficacy attributable to OIVIT (versus increased insulin exposure via conventional treatment routes or via increased medical attention). None of the studies provided robust health outcome data of interest to the Medicare program (versus efficacy data derived from surrogate markers). (See Outcomes of Greatest Interest) None of the studies provided robust safety data of interest to the Medicare program. This would include information on hypoglycemia and hypokalemia because of the acute effects of these metabolic conditions on older beneficiaries with cardiovascular disease and other co-morbidities. None of the studies were structured or analyzed to provide robust efficacy and safety data for risk/benefit analyses in various patient populations. Indeed, the high drop-out rate in the longest and largest study raises concerns about the long-term tolerability, long-term efficacy, and long-term safety of this invasive procedure. (Dailey 2000) Furthermore, none of the studies validated the role of respiratory quotients (RQ) ± urine urea nitrogen (UUN) measurements in assessing the effect of insulin dosing and/or determining subsequent insulin doses.
Some commenters disagreed with our review or interpretation of the evidence. In response, we believe that our review was exhaustive and that our interpretation is most consistent with a fair appraisal of the evidence base.
Although OIVIT has been claimed by some stakeholders to be standard therapy for glycemic control in a variety of diabetic patient populations and for control of diabetes-related complications, the available evidence does not support this. Other Federal health programs (Department of Defense, Indian Health Service, and the Veterans’ Affairs Health System) do not provide this therapeutic modality. No professional societies have included outpatient IV insulin therapy in their treatment guidelines or otherwise endorsed the therapeutic regimen.
Blue Cross/Blue Shield (California) and the Hayes Group have performed technology assessments of this therapeutic modality and concluded that the data did not support use of this treatment regimen. Many of the usual reviewers of technology, e.g., the Agency for Healthcare Research and Quality (AHRQ), the Cochrane Collaboration, and the National Institute for Health and Clinical Excellence (NICE), have not published technology assessments on outpatient intravenous insulin or metabolic cart measurements although they have conducted other assessments of diabetes-related technology and novel therapeutic regimens. The FDA has not approved or cleared the intravenous insulin treatment regimen, and no pumps have specifically been approved for this indication. The public commenters who advocated for CMS coverage did not provide scientific data that would support their position and many had conflicts of interest.
In summary, CMS does not believe that the available data from OIVIT studies have established its clinical benefit in any patient population for any medical condition. Furthermore, the evidence of record does not demonstrate that diagnostic tests for OIVIT efficacy, e.g., respiratory quotient (RQ) testing with or without urine urea nitrogen (UUN) testing, whether as single or multiple serial tests, provide information required or useful for diabetes management.
Therefore, CMS has determined that OIVIT is not reasonable and necessary. CMS has also determined that the components of an OIVIT regimen, including its diagnostic test components and supplies, are not reasonable and necessary when furnished in the context of the OIVIT regimen. As noted previously, this decision does apply the components of OIVIT when they are used outside of the OIVIT regimen.
IX. Conclusion
- The Centers for Medicare and Medicaid Services (CMS) has determined the following.
The evidence does not support a conclusion that outpatient intravenous insulin therapy improves health outcomes in Medicare beneficiaries. Therefore, CMS has determined that outpatient intravenous insulin therapy is not reasonable and necessary for any indication under section 1862(a)(1)(A) of the Social Security Act. Services comprising an Outpatient Intravenous Insulin Therapy regimen are nationally noncovered under Medicare when furnished pursuant to an outpatient intravenous insulin therapy regimen.
- Outpatient Intravenous Insulin Therapy (OIVIT) consists of an outpatient regimen of pulsatile or continuous intravenous infusion of insulin via any means, guided by the results of:
- measurement of respiratory quotient; and/or
- measurement of urine urea nitrogen (UUN); and/or
- measurement of arterial, venous or capillary glucose; and/or
- measurement of potassium concentration;
performed in scheduled recurring intermittent episodes.
This regimen is also sometimes termed Cellular Activation Therapy (CAT), Chronic Intermittent Intravenous Insulin Therapy (CIIIT), Hepatic Activation Therapy (HAT), Intercellular Activation Therapy (iCAT), Metabolic Activation Therapy® (MAT®), Pulsatile Intravenous Insulin Treatment (PIVIT), Pulse Insulin Therapy (PIT) and Pulsatile Therapy (PT).
Appendix A
General Methodological Principles of Study Design
(Section VI of the Decision Memorandum)
General Methodological Principles of Study Design
When making national coverage determinations, CMS evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that an item or service is reasonable and necessary. The overall objective for the critical appraisal of the evidence is to determine to what degree we are confident that: 1) the specific assessment questions can be answered conclusively; and 2) the intervention will improve health outcomes for patients.
We divide the assessment of clinical evidence into three stages: 1) the quality of the individual studies; 2) the generalizability of findings from individual studies to the Medicare population; and 3) overarching conclusions that can be drawn from the body of the evidence on the direction and magnitude of the intervention’s potential risks and benefits.
The methodological principles described below represent a broad discussion of the issues we consider when reviewing clinical evidence. However, it should be noted that each coverage determination has its unique methodological aspects.
Assessing Individual Studies
Methodologists have developed criteria to determine weaknesses and strengths of clinical research. Strength of evidence generally refers to: 1) the scientific validity underlying study findings regarding causal relationships between health care interventions and health outcomes; and 2) the reduction of bias. In general, some of the methodological attributes associated with stronger evidence include those listed below:
- Use of randomization (allocation of patients to either intervention or control group) in order to minimize bias.
- Use of contemporaneous control groups (rather than historical controls) in order to ensure comparability between the intervention and control groups.
- Prospective (rather than retrospective) studies to ensure a more thorough and systematical assessment of factors related to outcomes.
- Larger sample sizes in studies to demonstrate both statistically significant as well as clinically significant outcomes that can be extrapolated to the Medicare population. Sample size should be large enough to make chance an unlikely explanation for what was found.
- Masking (blinding) to ensure patients and investigators do not know to which group patients were assigned (intervention or control). This is important especially in subjective outcomes, such as pain or quality of life, where enthusiasm and psychological factors may lead to an improved perceived outcome by either the patient or assessor.
Regardless of whether the design of a study is a randomized controlled trial, a non-randomized controlled trial, a cohort study or a case-control study, the primary criterion for methodological strength or quality is the extent to which differences between intervention and control groups can be attributed to the intervention studied. This is known as internal validity. Various types of bias can undermine internal validity. These include:
- Different characteristics between patients participating and those theoretically eligible for study but not participating (selection bias).
- Co-interventions or provision of care apart from the intervention under evaluation (performance bias).
- Differential assessment of outcome (detection bias).
- Occurrence and reporting of patients who do not complete the study (attrition bias).
In principle, rankings of research design have been based on the ability of each study design category to minimize these biases. A randomized controlled trial minimizes systematic bias (in theory) by selecting a sample of participants from a particular population and allocating them randomly to the intervention and control groups. Thus, in general, randomized controlled studies have been typically assigned the greatest strength, followed by non-randomized clinical trials and controlled observational studies. The design, conduct and analysis of trials are important factors as well. For example, a well designed and conducted observational study with a large sample size may provide stronger evidence than a poorly designed and conducted randomized controlled trial with a small sample size. The following is a representative list of study designs (some of which have alternative names) ranked from most to least methodologically rigorous in their potential ability to minimize systematic bias:
- Randomized controlled trials
- Non-randomized controlled trials
- Prospective cohort studies
- Retrospective case control studies
- Cross-sectional studies
- Surveillance studies (e.g., using registries or surveys)
- Consecutive case series
- Single case reports
When there are merely associations but not causal relationships between a study’s variables and outcomes, it is important not to draw causal inferences. Confounding refers to independent variables that systematically vary with the causal variable. This distorts measurement of the outcome of interest because its effect size is mixed with the effects of other extraneous factors. For observational, and in some cases randomized controlled trials, the method in which confounding factors are handled (either through stratification or appropriate statistical modeling) are of particular concern. For example, in order to interpret and generalize conclusions to our population of Medicare patients, it may be necessary for studies to match or stratify their intervention and control groups by patient age or co-morbidities.
Methodological strength is, therefore, a multidimensional concept that relates to the design, implementation and analysis of a clinical study. In addition, thorough documentation of the conduct of the research, particularly study selection criteria, rate of attrition and process for data collection, is essential for CMS to adequately assess and consider the evidence.
Generalizability of Clinical Evidence to the Medicare Population
The applicability of the results of a study to other populations, settings, treatment regimens and outcomes assessed is known as external validity. Even well-designed and well-conducted trials may not supply the evidence needed if the results of a study are not applicable to the Medicare population. Evidence that provides accurate information about a population or setting not well represented in the Medicare program would be considered but would suffer from limited generalizability.
The extent to which the results of a trial are applicable to other circumstances is often a matter of judgment that depends on specific study characteristics, primarily the patient population studied (age, sex, severity of disease and presence of co-morbidities) and the care setting (primary to tertiary level of care, as well as the experience and specialization of the care provider). Additional relevant variables are treatment regimens (dosage, timing and route of administration), co-interventions or concomitant therapies, and type of outcome and length of follow-up.
The level of care and the experience of the providers in the study are other crucial elements in assessing a study’s external validity. Trial participants in an academic medical center may receive more or different attention than is typically available in non-tertiary settings. For example, an investigator’s lengthy and detailed explanations of the potential benefits of the intervention and/or the use of new equipment provided to the academic center by the study sponsor may raise doubts about the applicability of study findings to community practice.
Given the evidence available in the research literature, some degree of generalization about an intervention’s potential benefits and harms is invariably required in making coverage determinations for the Medicare population. Conditions that assist us in making reasonable generalizations are biologic plausibility, similarities between the populations studied and Medicare patients (age, sex, ethnicity and clinical presentation) and similarities of the intervention studied to those that would be routinely available in community practice.
A study’s selected outcomes are an important consideration in generalizing available clinical evidence to Medicare coverage determinations. One of the goals of our determination process is to assess health outcomes. These outcomes include resultant risks and benefits such as increased or decreased morbidity and mortality. In order to make this determination, it is often necessary to evaluate whether the strength of the evidence is adequate to draw conclusions about the direction and magnitude of each individual outcome relevant to the intervention under study. In addition, it is important that an intervention’s benefits are clinically significant and durable, rather than marginal or short-lived. Generally, an intervention is not reasonable and necessary if its risks outweigh its benefits.
If key health outcomes have not been studied or the direction of clinical effect is inconclusive, we may also evaluate the strength and adequacy of indirect evidence linking intermediate or surrogate outcomes to our outcomes of interest.
Assessing the Relative Magnitude of Risks and Benefits
Generally, an intervention is not reasonable and necessary if its risks outweigh its benefits. Health outcomes are one of several considerations in determining whether an item or service is reasonable and necessary. CMS places greater emphasis on health outcomes actually experienced by patients, such as quality of life, functional status, duration of disability, morbidity and mortality, and less emphasis on outcomes that patients do not directly experience, such as intermediate outcomes, surrogate outcomes, and laboratory or radiographic responses. The direction, magnitude, and consistency of the risks and benefits across studies are also important considerations. Based on the analysis of the strength of the evidence, CMS assesses the relative magnitude of an intervention or technology’s benefits and risk of harm to Medicare beneficiaries.
Appendix B
Bibliography
(Hardcopies available for weblinks.)
Abraira C, Colwell J, Nuttall F, Sawin CT, Henderson W, Comstock JP, et al. Cardiovascular events and correlates in the Veterans Affairs diabetes feasibility trial: Veterans Affairs cooperative study on glycemic control and complications in type II diabetes. Arch Intern Med 1997 January 27;157:181-187.
Abraira C, Duckworth W, McCarren M, Emanuele N, Arca D, Reda D, et al. Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Tria. J. Diabetes Complications 2003 Nov-Dec;17(6):314-322.
Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study Group, Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. NEJM 2008 Jun 12;358(24):2545-2559.
Advanced Diabetes Treatment Center. Online at:
http://www.adtcusa.com/DOR.htm; and
http://www.adtcusa.com/managementteam.html; and
http://drnachlas.com/dr-nachlas.html; and
http://www.ccs.fau.edu/~Tuller/publ.html; and
http://www.adtcusa.com/departmentofresearch.html; and
http://www.secinfo.com/d11Eeb.4f89d.b.htm
(See Diabetex. See SEC.)
Agency for Healthcare Research and Quality (AHRQ).
(The AHRQ does not have any guidelines that address the use of outpatient intravenous insulin therapy or the use of calorimetry in diabetic patients.)
AHRQ External Technology Assessments
i. Balk E, Teplinsky E, Trikalinos T, Chew P, Chung M, Lau J, Pittas A. Applicability of the Evidence Regarding Intensive Glycemic Control and Self-Monitored Blood Glucose to Medicare Patients with Type 2 Diabetes. September 10, 2007.
ii. Holohan TV. Simultaneous Pancreas-Kidney and Sequential Pancreas-After-Kidney Transplantation. August 1995.
iii. Hotta SS. Isolated Pancreas Transplantation. August 1995.
iv. Matchar DB, Keefe FJ, McCrory DC, Scipio CD, Cooper K, Kolimaga JT, Huntington AC. Use of Behavioral Therapies for Treatment of Medical Disorders Part 1 Impact on Management of Patients with Diabetes Mellitus. May 9, 2004.
v. Matchar DB, McCrory DC, Samsa GP, Lobaugh B, Liu K. Point of Care Testing of Hemoglobin A1c. August 30, 2005.
(See Cochrane. See NICE.)
Aetna. Clinical Policy Bulletin: Intermittent Intravenous Insulin Therapy. Number: 0742. Online at: http://www.aetna.com/cpb/medical/data/700_799/0742.html
Agency for Healthcare Research and Quality. Effective Health Care: Comparative effectiveness and safety of oral diabetes medications for adults with type 2 diabetes. Executive Summary Number 8. Online at: http://effectivehealthcare.ahrq.gov/repFiles/OralExecutiveSummary.pdf
Agius L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochem J 2008 Aug 15;414(1):1-18.
Agius L. New hepatic targets for glycaemic control in diabetes. Best Pract Res Clin Endocrinol Metab 2007 Dec;21(4):587-605.
Agius L. Targeting hepatic glucokinase in type 2 diabetes: Weighing the benefits and risks. Diabetes 2009 Jan;58(1):18-20.
Aguis L, Peak M, VanSchaftingen E. The regulatory protein of glucokinase binds to the hepatocyte matrix, but, unlike glucokinase, doses not translocate during substrate stimulation. Biochem J 1995 Aug 1;309 (Pt 3):711-713.
Alfonzo AV, Isles C, Geddes C, Deighan C. Potassium disorders-clinical spectrum and emergency management. Resuscitation 2006 Jul;70(1):10-25.
Allon M, Copkney C. Albuterol and insulin for treatment of hyperkalemia in hemodialysis patients. Kidney International 1990;38:869-872.
American Association of Clinical Endocrinologists (AACE)
(The AACE does not have any guidelines that address use of this therapeutic modality or the use of calorimetry in diabetic patients.)
AACE 9/2009 letter to CMS. (Hardcopy available.)
AACE Medical Guidelines for the Clinical Practice for the Management of Diabetes Mellitus. AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. Chair HW. Rodbard, 2007.
American Association of Clinical Endocrinologists Medical Guidelines for the Clinical Use of Dietary Supplements and Nutraceuticals. AACE Nutrition Guidelines Task Force. Chair JL Mechanick, 2003.
American Association of Clinical Endocrinologists/American College of Endocrinology’s guidelines for the Management of DM Medical Guidelines for Clinical Practice for the Management of Diabetes Mellitus 2007;13(Suppl 1):1-66. Online at: http://www.aace.com/pub/pdf/guidelines/DMGuidelines2007.pdf
American Association for Respiratory Care (AARC)
(The AARC does not have any guidelines that address use of this therapeutic modality or the use of calorimetry in diabetic patients.)
AARC Clinical Practice Guideline: Metabolic measurement using indirect calorimetry during mechanical ventilation – 2004 revision & update. Respiratory Care 2004 September:49(9):1073-1079. Online at: http://www.rcjournal.com/cpgs/pdf/09.04.1073.pdf
American College of Cardiology (ACC)
(The ACC has guidelines for cardiac disease. The ACC does not have any guidelines that address use of the outpatient intravenous insulin therapeutic modality or the use of calorimetry in diabetic patients.)
American College of Chest Physicians (ACCP)
(The ACCP does not have any guidelines that address use of the outpatient intravenous insulin therapeutic modality or the use of calorimetry in diabetic patients.)
ACCP has guidelines for cardiovascular disease and the use of gas exchange devices as part of exercise tolerance testing.
American College of Chest Physicians (ACCP) and American Thoracic Society (ATS)
The ACCP/American Thoracic Society (ATS) has guidelines for cardiopulmonary disease and some aspects of nutrition.
ATS/ACCP Statement: Cardiopulmonary Exercise Testing (Corrected Version) [Original accepted by Society 11/1/01]. (The joint guidelines with the ATS address the importance of calibrating equipment and the use of the gas exchange devices as part of exercise tolerance testing.)
American College of Chest Physicians/American Thoracic Society/ (ACCP/ATS). Medically Unlikely Edit Letter July 30, 2009. (See CCI-Rosen. See Parrish.)
American College of Physicians (ACP) (The ACP does not have any guidelines that address use of this therapeutic modality or the use of calorimetry in diabetic patients.)
The ACP guidelines for diabetes.
Glycemic Control and Type 2 Diabetes Mellitus: The Optimal Hemoglobin A1c Targets. A Guidande Statement from the American College of Physicians 10/28/06. A Qaseem, S Vijan, V Snow, J Cross, K Weiss, D Owens for the Clinical Efficacy Assessment Subcommittee of the American College of Physicians.
Guidelines on Lipid Control in Type 2 Diabetes 7/03. V Snow, M Aronson, E Hombake, C Mottur-Pilson, K Weiss for the Clinical Efficacy Assessment Subcommittee of the American College of Physicians
Pharmacologic Lipid-Lowering Therapy in Type 2 Diabetes Mellitus: Background Paper for the American College of Physicians (evidence review) 4/20/2004.
American Diabetes Association. Diabetes Mellitus. What is the impact of diabetes? Online at: http://www.medicinenet.com/diabetes_mellitus/article.htm
American Diabetes Association (ADA). (The ADA has guidelines and publications about diabetes and diabetes management for both patients and professionals. The ADA does not have any guidelines that address use of outpatient intravenous insulin or the use of calorimetry in diabetic patients.)
Professional Guidelines
All About Diabetes. Online at:
http://www.diabetes.org/about-diabetes.jsp
2008 American Diabetes Association Clinical Guidelines. Online at:
http://www.diabetesincontrol.com/results.php?storyarticle=5576
Summary of Revisions for the 2008 Clinical Practice Recommendations. Online at:
http://care.diabetesjournals.org/content/31/Supplement_1/S3.extract
ADA Standards of Medical Care in Diabetes – 2009. Online at:
http://care.diabetesjournals.org/content/32/Supplement_1/S13.full
Executive Summary Standards of Medical Care in Diabetes – 2009. Online at:
http://care.diabetesjournals.org/content/32/Supplement_1/S6.full.pdf+html
Committee Reports and consensus Statements. Online at: http://care.diabetesjournals.org/content/32/Supplement_1/S96.full.pdf+html
Diabetes Care: Insulin Administration ADA Position Statement. Diabetes Care 2004 Jan;27 Suppl (1):S106-S109. Online at: http://care.diabetesjournals.org/content/27/suppl_1/s106.full.pdf+html
Hyperglycemic crises inpatients with diabetes mellitus. Diabetes Care 2003 January;26(1):S109-S117.
List of Position Statements. Online at: http://care.diabetesjournals.org/content/32/Supplement_1/S98.full.pdf+hrml
Nutrition Recommendations and interventions for diabetes: A position statement of the ADA. Online at:
http://care.diabetesjournals.org/content/31/Supplement_1/S61.full.pdf+html
Position Statement. Online at: http://care.diabetesjournals.org/content/32/Supplement_1/S98.full.pdf+html
Standards in Medical Care in Diabetes–2008. Online at:
http://care.diabetesjournals.org/content/31/Supplement_1/S12.extract
Standards of Medical Care in Diabetes–2009. Diabetes Care Jan 2009. 32 Suppl (1):S13-S61. Online at:
http://care.diabetesjournals.org/content/31/Supplement_1/S12.full.pdf+html
Summary of Revisions for the 2009 Clinical Practice Recommendations. Online at: http://care.diabetesjournals.org/content/32/Supplement_1/S3.full.pdf+html
Treatment Guidelines Patient summary. Online at:
http://www.goinsulin.com/why-insulin/ada-guidelines.aspx
Position Statement: Unproven Therapies. Diabetes Care 2004 January;27 Suppl (1):S135.
ADA Richard Kahn, PhD expert comment on September 18, 2009 on Outpatient Intravenous Insulin Treatment. (Hardcopy available.)
Professional Publications
The Practical Insulin – A Handbook for Prescribers. Online at:
http://store.diabetes.org/products/product_details.jsp?StoreJSESSIONID=KD5G22DfhAF4QOU02xOuv2XQdhxP2tR1bSnsjoIzrGWHwToXdcJ3!-152276538!-1407451110!7005!8005&PRODUCT<>prd_id=845524441763991&FOLDER<>folder_id=2534374302023945&bmUID=1245952326393
Intensive Diabetes Management Updates, 4th Edition. Online at:
http://store.diabetes.org/products/product_details.jsp?StoreJSESSIONID=KpVrp0Huaa4I1rSOPCddjnPGR3TuwE4V70NHc2gEM1thIDwswrf2!-416284431!-1407451110!7005!8005&PRODUCT<>prd_id=845524441764396&FOLDER<>folder-id=2534374302023863&bmUID=1244222827433
Practical insulin – A handbook for prescribers. 2002. Library of Congress Cataloging-in-Publication Data. United States of America. CMS copy (WK 39 P895 2002) on loan (July 2009) from the Baltimore Medical Center, 10 N. Green Street, Baltimore, MD 21201.
On Insulin Pumps. Online at:
http://www.diabetes.org/type-1-diabetes/insulin-pumps.jsp
Technical Reviews. Online at: http://care.diabetesjournals.org/content/32/Supplement_1/S95.full.pdf+html
Related Patient Materials
ADA Treatment Guidelines patient Summary. Online at:
http://www.goinsulin.com/why-insulin/ada-guidelines.aspx
About Insulin and other Drugs – ADA. Online at:
http://www.diabetes.org/type-2-diabetes/insulin.jsp
Diabetes Statistics. Online at:
http://www.diabetes.org/diabetes-statistics.jsp
American Dietetic Association (ADA)
(The ADA does not have any guidelines that address use of outpatient intravenous insulin or the use of outpatient calorimetry in diabetic patients.)
The ADA guidelines for nutrition in diabetic patients
ADA Diabetes Type 1 and 2 Evidence-based Nutrition Practice Guideline for Adults 2006 GET. Online at:
http://www.adaevidencelibrary.com/topic.cfm?cat=3252
The ADA guideline for nutrition during critical illness
(The guideline discusses metabolic cart measurements in the hospital setting.) Online at:
http://www.guideline.gov/summary/summary.aspx?ss=15&doc_id=12818&string=
American Geriatric Society (AGS)
(The AGS does not have any guidelines that address use of intravenous insulin or the use of calorimetry in diabetic patients.)
AGA guidelines for diabetes care
Guidelines for Improving the Care of the Older Person with Diabetes Mellitus-2003. California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Online at:
http://www.americangeriatrics.org/products/positionpapers/JAGSfinal05.pdf
Guidelines for improving the care of the older person with diabetes mellitus: California Healthcare Foundation/American Geriatrics Society Panel on improving care for elders with diabetes. JAGS 2003;51(5)(Suppl 1):S265-S280. Online at: http://www.americangeriatrics.org/products/positionpapers/JAGSfinal05.pdf
American Heart Association (AHA)
(The AHA does not have any guidelines that address use of intravenous insulin or the use of calorimetry in diabetic patients.)
The AHA guideline for clinical exercise testing
Guidelines for Clinical Exercise Testing Laboratories-1995. IL Pina, GJ Balady, P Hanson, AJ Labovitz, DW Madonna, J Myers. Online at:
http://circ.ahajournals.org/cgi/content/full/91/3/912
The AHA Joint Scientific Statement with the American Diabetes Association
Primary Prevention of Cardiovascular Diseases in People with Diabetes Mellitus-2006. JB Buse, HN ginsberg, GL Bakris, NG Clark, F Costa, R Eckel, V Fonseca, HC Gerstein, S Grundy, RW Nesto, MP Pignone, J Plutzky, D Porte, R Redberg, KF Stitzel, NJ Stone. Online at:
http://circ.ahajournals.org/cgi/content/full/115/1/114
American Medical Association (AMA)
Current Procedural Terminology (CPT) 2008: Professional Edition 2008.
Current Procedural Terminology Assistant: Your practical guide to current coding. Pulmonary Function Testing. Jan 1999. 9;(1). Online at: http://reimbursement.respironics.com/downloads/pulmon.pdf
Physician Resources/Solutions. Managing your practice coding/billing-insurance/cpt/applying cpt codes. Online at:
http://www.ama-assn.org/ama/no-index/physician-resources/18192.shtml
Resource-Based Relative Value Scale (RBRVS) Update Committee. Online at:
http://www.ama-assn.org/ama/pub/physician-resources/solutions-managing-your-practice/coding-billing-insurance/medicare/the-resource-based-relative-value-scale.shtml
American Medical Association (AMA) or the Centers for Medicare and Medicaid Services (CMS). Requests for new codes. Submit to:
http://www.bing.com/search?srch=105&FORM=IE7RE&q=American+Medical+Association+(AMA)+or+the+Centers+for+Medicare+and+Medicaid+Services+(CMS).+Requests+for+new+codes.
American Society of Consultant Pharmacists. Change in Medicare policy on reimbursement for fingerstick blood glucose tests in nursing facility residents. Federal Register. 71 FR 69624 Final Rule. Department of Health and Human Services. 2006 Dec 1; 42 CFR Parts 405, 410, 411, 414, 415 and 424. Proposed Rule CMS-1321-FC. Online at: http://www.clinicalreimbursement.com/MyFiles/PDF/Blood glucose 11_06.pdf
American Thoracic Society (ATS)
(The ATS does not have any guidelines that address use of the outpatient intravenous insulin therapeutic modality or the use of calorimetry in diabetic patients.)
The ATS guidelines for cardiopulmonary disease and some aspects of nutrition
ATS/ACCP Statement: Cardiopulmonary Exercise Testing (Corrected Version) (original accepted by Society 3/10/01). Am J Respir Crit Care Med 2003;167:211-277.
(The joint guidelines address the importance of calibrating equipment and the use of the gas exchange devices as part of exercise tolerance testing.)
Statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002:166:111-117. Online at:
http://www.thoracic.org/sections/publications/statements/pages/pfet/sixminute.html
American Thoracic Society/American College of Chest Physicians (ATS/ACCP). Medically Unlikely Edit Letter. July 30, 2009. (See CCI-Rosen. See Parrish.)
Andralojc KM, Mercalli A, Nowak KW, Albarello L, Calcagno R, Luzi L, et al. Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia 2009;52:486-493.
Aoki Diabetes Research Institute (ADRI). Online at:
http://www.adri.org/faqs.html; and
http://www.adri.org/news.html; and
http://www.adri.org/injunction.html; and
http://www.plol.org/Pages/Login.aspx?d=2FZNkJAGVDaZ8SxMWyXqng==&l=Cases.
(See SEC. See Diabetex.)
Aoki TT. Patents. Online at:
http://www.wipo.int/pctdb/en/wo.jsp?wo=1985002544; and http://www.patentbuddy.com/Inventor/Patents/Aoki/Thomas/5764214; and http://www.freshpatents.com/Thomas-T-Aoki-Sacramento-invdira.php
http://www.freepatentsonline.com/EPO165973B.html
Aoki TT. Response to Blue Shield. (Submitted April 20, 2009 with public comments via mail from Michael Bradley, Clinic Manager, Aoki Diabetes Research Institute.)
Aoki TT, Assal J-P, Manzano FM, Kozak GP, Cahill GF. Plasma and cerebrospinal fluid amino acid levels in diabetic ketoacidosis before and after corrective therapy. Diabetes 1975 May;24(5):465-467.
Aoki TT, Benbarka MM. Type I diabetes: The ‘sleeping liver’ hypothesis and its clinical implications. Mod Med 1992 Oct;60:73-76.
Aoki TT, Benbarka MM, Oklmura MC, Arcangeli MA, Walter RM, Wilson LD, et al. Long-term intermittent intravenous insulin therapy and type 1 diabetes mellitus. Lancet 1993 Aug 28;342(8870):515-518.
Aoki TT, Grecu EO, Arcangeli MA. Chronic Intermittent Intravenous Insulin Therapy (CIIIT) corrects orthostatic hypotension of diabetes. Am J Med 1995c Dec;99(6):683-684.
Aoki TT, Grecu EO, Arcangeli MA. Chronic Intermittent Intravenous Insulin Therapy (CIIIT) reduces antihypertensive medication requirements in hypertensive type 1 diabetic patients (IDDM). Diabetes 1993. 204A.
Aoki TT. Grecu EO, Arcangeli MA. The effect of Chronic Intermittent Intravenous Insulin Therapy (CIIIT) on the progression of nephropathy inpatients with type 1 diabetes mellitus (IDDM). Clin Res 1993. 41:109A.
Aoki TT, Grecu EO, Arcangeli MA, Benbarka MM, Prescott P,Ahn JH. Chronic Intermittent Intravenous Insulin Therapy (CIIIT): A new frontier in diabetes therapy. Diabetes Technology and Therapeutics Review 2001 Spring;3(1):111-123.
Aoki TT, Grecu EO, Arcangeli MA, Meisenheimer R. Effect of intensive insulin therapy on abnormal circadian blood pressure pattern inpatients with type 1 diabetes mellitus. Online J Curr Clin Trials 1995b Dec 15;Doc No 19.
Aoki TT, Grecu EO, Gollapudi GM, Barber AR Arcangeli MA, Benbarka MM, et al. Effect of intensive insulin therapy on progression of overt nephropathy inpatients with type 1 diabetes mellitus. Endocr Pract 1999 Jul/Aug;5(4):174-178.
Aoki TT, Grecu EO, Prendergast JJ, Arcangeli MA, Meisenheimer R. Effect of chronic intermittent intravenous insulin therapy on antihypertensive medication requirements in IDDM subjects with hypertension and nephropathy. Diabetes Care 1995a Sept;18(9):1260-1265.
Aoki TT, Muller WA, Brennan MF, Cahill GF JR. Blood cell and plasma amino acid levels across forearm muscle during a protein meal. Diabetes 1973 Oct;22(10):768-775.
Aoki TT, Vlachokosta FV, Foss MC, Meistas MT. Evidence for restoration of hepatic glucose processing in type 1 diabetes mellitus. J Clin Invest 1983 Apr;71(4):837-839.
Aoki TT, White RD. Initiating insulin inpatients with type 2 diabetes. J Fam Pract 2007 Aug;56(Suppl 8):S12-20.
Aron D, Pogach L. Transparency Standards for Diabetes Performance Measures. JAMA 2009 January 14;301(2): 210- 212.
Ashcroft FM. K ATP channels and insulin secretion: a key role in health and disease. Biochemical Society. Transactions 2006. 34;(Part 2):243-246.
Ashcroft FM, Gribble FM. ATP-sensitive K+ channels and insulin secretion: their role in health and disease. Diabetologia 1999. 42:903-919.
Assal JP, Aoki TT, Manzano FM, Kozak GP. Metabolic effects of sodium bicarbonate in management of diabetic ketoacidosis. Diabetes 1974 May;23(5):405-411.
Axelrod L, Shulman GI, Blackshear PJ, Bornstein W, Roussell AM, Aoki TT. Plasma level of 13.14-dihydro-15-keto-PGE2 in patients with diabetic ketoacidosis and in normal fasting subjects. Diabetes 1986 Sep;35(9):1004-1010.
Balanced Budget Act of 1997, H.R. 2015. Provides expanded coverage of glucose monitors and test strips for all diabetics. Implementation July 1, 1998. Online at: http://thomas.loc.gov/cgi-bin/query/z?c105:H.R.2015.ENR
Balkin M, Mascioli C, Smith V, Alnachawati H, Mehrishi S, Saydain G, et al. Research Article. Achieving durable glucose control in the intensive care unit without hypoglycaemia: a new practical IV insulin protocol. Diabetes Metab Res Rev 2007. 23:49-55.
Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008 Jan;31(Suppl 1):S61-78.
Baron AD, Brechtel G, Wallace P, Edelman SV. Fasting decreases rates of noninsulin-mediated glucose uptake in man. J Clin Endocrinol Metab 1988 Sep;67(3):532-540.
Baron AD, Brechtel G, Wallace P, Edelman S. Rates and tissue sites of non-insulin and insulin-mediated glucose uptake in humans. Am J Physiol 1988 Dec;255(6Pt1):E769-774.
Baron AD, Wallace P, Brechtel G. In vivo regulation of non-insulin mediated tend insulin-mediated glucose uptake by cortisol. Diabetes 1987 Nov;36(11):1230-1237.
Bartlett RG. Factors affecting the respiratory quotient. Thesis – University of Maryland. Transcript. Library of Congress. 1952.
Basu A, Basu R, Shah P, Vella A, Johnson CM, Nair KS, et al. Effects of type 2 diabetes on the ability of insulin and glucose to regulate splanchnic and muscle glucose metabolism: evidence for a defect in hepatic glucokinase activity. Diabetes 2000 Feb;49(2):272-283.
Benade AJ, Jansen CR, Rogers GG, Wyndham CA, Strydam NB. The significance of an increased RQ after sucrose ingestion during prolonged aerobic exercise. Pfagers Arch 1973 Aug 27;342(3):199-206.
Benbarka MM, Presott PT, Aoki TT. Practical guidelines on the use of insulin lispro in elderly diabetic patients. Drugs Aging 1998 Feb;12(2):103-113.
Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab 2000 Nov;11(9):351-356.
Bergman N, Vellar ID. Potential life-threatening variations of drug concentrations in intravenous infusion systems: Potassium chloride, insulin, and heparin. Med J Aust 1982 Sep 18;2(6):270-272.
Björklund AO, Adamson UK, Lins PS, Westgren LM. Diminished insulin clearance during late pregnancy inpatients with type1 diabetes mellitus. Clin Science 1998;95:317-323.
Blue Cross Blue Shield of Arkansas. Policy Number 2005001. Chronic Intermittent Intravenous Insulin Therapy (CIIIT). Initiated: January 2005. Last Reviewed: May 2007. Online at http://www.arkansasbluecross.com/members/report.aspx?policyNumber=200501.
Blue Cross Blue Shield of California. Technology Assessment 2001.
Blue Cross Blue Shield of Florida Policy number: 01-99000-04. Chronic Intermittent Intravenous Insulin Therapy (CIIIT) [Pulsatile Intravenous Insulin Therapy (PIVIT), Hepatic Activation, Metabolic Activation]. Effective November 15, 2002. Online at: http://mcgs.bcbsfl.com/?doc=Chronic Intermittent Intravenous Insulin Therapy (CIIIT)
Blue Cross Blue Shield of Idaho policy MP 2.01.43. Chronic Intermittent Intravenous Insulin Therapy (CIIIT). Considered investigational. Online at: http://www.bcidaho.com/providers/medical_policies/med/mp_20143.asp
Blue Cross Blue Shield of Massachusetts policy #322. Insulin Delivery Devices and Excluded Services. Do not cover Chronic Intermittent Intravenous Insulin Therapy (CIIIT) or Pulsatile IV Insulin Therapy (PIVIT) due to lack of efficacy. Online at: http://www.bluecrossma.com/common/en_US/medical_policies/332 Insulin Delivery Devices prn.pdf
Blue Cross Blue Shield of Mississippi policy # 2.01.43. Chronic Intermittent Insulin Therapy (CIIT). Non covered as investigational. Online at: https://www.bcbsms.com/index.php?q=member-medical-policy-search.html&action=viewPolicy&path=/policy/emed/Chronic Intermittent Intravenous Insulin Therapy (CIIIT).html
Blue Cross Blue Shield of North Carolina policy #MED1243. Insulin Therapy, Chronic Intermittent Intravenous. Non covered as investigational. Online at: http://www.bcbsnc.com/assets/services/public/pdfs/medicalpolicy/insulin_therapy_chronic_intermittent_intravenous.pdf
Blue Cross and Blue Shield of Washington (Regence Blue Shield Newsletter). Online at: http://www.wa.regence.com/provider/library/newsletters/index.html
Blue Shield’s review of Hepatic Activation. (Submitted April 20, 2009 with public comments via mail from Michael Bradley, Clinic Manager, Aoki Diabetes Research Institite).
Blundell JE, Coding J, King NA. Differences in postprandial responses to fat and carbohydrate loads in habitual high and low fat consumers (phenotypes). Br J Nutr 2002 Aug;88(2):125-132.
Bohannon NJ. Insulin delivery using pen devices. Simple-to-use tools may help young and old alike. Postgrad Med 1999 Oct 15;106(5):57-8, 61-4, 68.
Braithwaite S. Detection and management of diabetes mellitus during glucocorticoid therapy of nocrine disease. In: Meikle AW, ed. Endocrine Replacement Therapy in Clinical practice. Totowa, NJ: Humana Press, Inc, 2003. 251-272.
Braithwaite S, Buie M, Thompson C, Baldwin D, Oertel M, Robertson BA, et al. Hospital hypoglycemia: not only treatment but also prevention. Endocr Pract 2004 Mar-Apr;10 Suppl (2):89-99.
Brandenburg D. History and diagnostic significance of C-peptide. Exp Diabetes Res 2008;576862-576869.
Branson RD. The measurement of energy expenditure: instrumentation, practical considerations, and clinical application. Resp Care 1990. 35:640-656.
Bratusch-Marrain PR, Komjati M, Waldhäusl WK. Efficacy of pulsatile versus continuous insulin administration on hepatic glucose production and glucose utilization in type 1 diabetic humans. Diabetes 1986 Aug;35(8):922-926.
Bratusch-Marrain PR, Komjati M, Waldhäusl WK. Pulsatile insulin delivery: physiology and clinical implications. Diabet Med 1987 May-Jun;4(3):197-200.
Breen PH, Isserles SA, Taitelman UZ. Non-steady state monitoring by respiratory gas exchange. J of Clinical Monitoring and Computing 2000. 16:351-360.
Brogden RN, Heel RC. Human insulin. A review of its biological activity, Pharmacokinetics and therapeutic use. Drugs 1987 Sep;34(3):350-371.
Brown JG (Inspector General) Office of the Inspector General (OIG). Blood glucose test strips: Marketing to Medicare beneficiaries. Department of Health and Human Services. 2000 Jun; OEI-03-98-00231. Online at:
http://oig.hhs.gov/oei/reports/oei-03-98-00231.pdf
Bruni B, Ricci C, Giolitti A, Osenda M. D’Alberto M. Turco GL. Nuclear Medicine: Insulin antibody production studied quantitatively with a modified radioimmunoassay technique and with radioimmuno-electrophoresis inpatients treated with monocomponent (MC) insulin. J. Nucl. Boil. Med 1973. 17(3):123.
Burge MR, Schade DS. Current Therapies for Diabetes: Insulins. Endocrinol Metab Clin North Am 1997 Sep;26(3):575-598.
Cahill GF Jr, Aoki TT. How metabolism affects clinical problems. Med Times 1970 Oct;98(10):106-122.
Cahill GF Jr, Aoki TT, Ruderman NB. Ketosis. Trans Am Clin Climatol Assoc 1973;84:184-202.
Cahill GF Jr, Rossini AA, Aaoki TT. Metabolic effects of insulin in normal and diabetic man (short review). Endocrinol Exp 1974 Jun;8(2):89-96.
California Court of Appeals upholding the Superior Court decision. (Submitted April 20, 2009 with public comments via mail from Michael Bradley, Clinic Manager, Aoki Diabetes Research Institute.) (Hardcopy available.)
California Healthcare Foundation/American Geriatrics Society Panel on improving Care for Elders with Diabetes: Guidelines for Improving the Care of the Older Person with Diabetes Mellitus. JAGS 2003;51:S265-S280.
California Physicians’ Service Blue Shield v. Aoki Diabetes Research Institute. Super. Ct. No. CGC-03-419872). Filed Jun 17, 2008. Online at: http://www.google.com/search?q=California+Physicians'+Service+v+Aoki+Diabetes+Research+Institute
California Public Employees’ Retirement System (CALPERS): Board of Administration. In the matter of the consolidated appeals of denial of coverage for hepatic activation treatment of: Names withheld (5). Case No.: 3490-5, 3490-3, 3490-2, 3490-1, 3490-4 and 3490-6. (Hardcopy available.)
CALPERS Court Ruling 2002. Online at:
F:\My Projects\Diabetes\Metabolic Activated Therapy CAG 00410N\NCA\Calpers Decision\CalPers Decision 4 30 02.pdf
CALPERS Judgement. Online at:
F:\My Projects\Diabetes\Metabolic Activated Therapy CAG 00410N\NCA\Calpers Decision\judgment.pdf
CALPERS Legal Issue 6. Online at:
F:\My Projects\Diabetes\Metabolic Activated Therapy CAG 00410N\NCA\Calpers Decision\Order Legal Issue 6.pdf
CALPERS Order Re Legal Issues. Online at:
F:\My Projects\Diabetes\Metabolic Activated Therapy CAG 00410N\NCA\Calpers Decision\Order Re Legal Issues.pdf
CALPERS Proposed Decision 1.17.02. Online at:
F:\My Projects\Diabetes\Metabolic Activated Therapy CAG 00410N\NCA\Calpers Decision\CalPers Proposed Decision 1 17 02.pdf
Canadian Diabetes Association (CDA).
(The CDA does not have any guidelines that address use of this therapeutic modality or the use of calorimetry in diabetic patients.)
(The CDA has a single comprehensive guideline for diabetes.)
Canadian Diabetes Association 2008 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. 2008 September;32 Suppl (1):S1-S201. http://www.diabetes.ca and http://www.diabetes.ca/for-professionals/resources/2008-cpg/
Canadian Diabetes Association Clinical Practice Guidelines Expert Committee.
The most pertinent chapter(s) include:
Diabetes in the Elderly. G Meneilly, D Tessier (initial draft);
Insulin Therapy in Type 1 Diabetes.A Cheng, A Hanna, T Kader, C Richardson;
Pharmacologic Management of Type 2 Diabetes.W Harper, A Hanna, V Woo, K Dawson, JF Yale, L MacCallum, M Clement, S Simpson,M Hopkins (initial draft);
In-hospital management of Diabetes.A Edwards, A Cheng, M Clement, a Hanna, R Houlden, J James (initial draft)
The CDA guideline section on intravenous insulin is delineated in the "In-hospital" chapter.
"Role of Intravenous Insulin Infusion. Intravenous (IV) insulin infusion therapy should be considered during critical illness, or other illness requiring prompt glycemic control, or prolonged fasting (NPO status) (7). IV insulin infusion therapy should be administered only where frequent blood glucose (BG) monitoring and close nursing supervision are possible. Staff education is a critical component of the implementation of an IV insulin infusion protocol. IV insulin protocols should take into account the current and previous BG levels (and, therefore, the rate of change), and the patient’s usual insulin dose."
Carmel PW, Araki S, Ferin M. Pituitary stalk portal blood collection in rhesus monkeys: evidence for pulsatile release of gonadotropin-rleasing hormone (GnRH). Endocrinol 1976 Jul;99(1):243-248.
Caumo A, Luzi L. First-phase insulin secretion: does it exist in real life? Considerations on shape and function. Am J Physiol Endocrinol Metab 2004 Sep;287(3):E371-E385.
Cavalcanti AB, Silva E, Pereira AJ, Caldeira-Filho M, Almeida FP, Westphal GA, et al. A randomized controlled trial comparing a computer-assisted insulin infusion protocol with a strict and conventional protocol for glucose control in critically ill patients. J of Critical Care. 2009. Article in Press.
Cefalu WT. Evolving strategies for insulin delivery and therapy. Drugs 2004. 64(11):1149-1161.
Centers for Medicare and Medicaid Services (CMS) Program Memorandum Intermediaries/Carriers. Glucose monitoring. Transmittal AB-00-108; Change Request 1362, December 1, 2000. Online at:
http://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/Downloads/AB00108.pdf
Chaiken RL. EXUBERA® (insulin human [rDNA origin] Inhalation Powder) Labeling Update letter. April 9, 2008.
Chan E, Montgomery PA. Administration of insulin by continuous ambulatory peritoneal dialysis. Pharmacotherapy 1993 Sep-Oct;13(5):455-460.
Chance, RE, Frank BH. Research, development, production, and safety of biosynthetic human insulin. Diabetes Care 1993. 16(Suppl 3):133-142.
Cheatham B. GLUT4 and Company: SNAREing roles in insulin-regulated glucose update. TEM 2000;11(9):356.
Chemical and Engineering News. Lilly drops inhaled insulin. Move adds to growing list of failures for technology. Pharmaceuticals. 2008 March 17;86(11):9. Online at: http://pubs.acs.org/cen/news/86/i11/8611notw2.html.
Cherrington AD, Sindelar D, Edgerton D, Steiner K, McGuinnes OP. Physiological consequences of phasic insulin release in the normal animal. Diabetes 2002 Feb;51(Suppl 1):S103-S108.
Cheung AT, Ramanujam S, Greer DA, Kumagai LF, Aoki TT. Microvascular abnormalities in the bulbar conjunctiva of patients with type 2 diabetes mellitus. Endocrin Pract 2001 Sep-Oct;7(5)358-363.
Chiarelli F, Verrotti A, Catino M, Sabatino G, Pinelli L. Hypoglycaemia in children with type 1 diabetes mellitus. Acta Paediatr 1999. Jan;427(Suppl):31-34.
CIGNA A41337: Chronic Intermittent Intravenous Insulin Therapy (CIIIT). Article clarifying noncoverage of MAT. Applies to ID, NC, and TN. Online at: http://www.cms.gov/MVD/m_a.asp?id=41337&ver=4&cid=05535
Clark C, Newgard CB. Chapter 5 Hepatic Regulation of Fuel Metabolism. [Saltiel AR, Pessin JE. Editors.] Mechanisms of insulin action. Medical Intelligence Unit. Springer Science+Business Media, New York, New York U.S.A. 2007:90-103. Online at: http://books.google.com/books?id=HUi95xaBBDYC&pg=PA52&lpg=PA52&dq=Mechanisms+of+Insulin+Action&source=bl&ots=zzlqAD5T5B&sig=FzjJ2MxPxpb48D852l1nZN0qYDg&hl=en&ei=4l6mSqbpMcOmlAeGnYGQBA&sa=X&oi=book_result&ct=result&resnum=3#v=onepage&q=Chapter by Clark&f=false
Clemens AH, Hough DL, D’Orazlo PA. Development of the Biostator® Glucose Clamping Algorithm. Clin Chem 1982. 28(9):1899-1904.
ClinicalTrials.gov. (Personal communication with Dr. Zarin. March 2009.) Online at: http://clinicaltrials.gov/ct2/results?term=pulsatile+insulin+ for: NCT00228891; NCT00228904; NCT00287651; NCT00539435; NCT00361907; NCT00228878; NCT00539409; NCT00228865; and NCT00594152
Cochrane Collaboration External Technology Assessments
i.Farrar D, Tuffnell DJ, West J. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. July 18, 2007.
ii.Goudswaard AN, Furlong NJ, Valk GD, Stolk RP, Rutten GEHM. Insulin monotherapy versus combinations of insulin with oral hypoglycaemic agents inpatients with type 2 diabetes mellitus. October 18, 2004.
iii.Horvath K, Jeitler K, Berghold A, Ebrahim SH, Gratzer TW, Plank J, Pieber TR, Siebenhofer A. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. April 18, 2007.
iv.Onady GM, Stolfi A. Insulin and oral agents for managing cystic fibrosis-related diabetes. July 20, 2005. Update: November 3, 2008.
v.Richter B, Neises G. Human insulin versus animal insulin in people with diabetes mellitus. July 22, 2002. Update: July 31, 2004.
vi. Siebenhofer A, Plank J, Berghold A. Jeitler K, Horvath K, Narath M. Gfrerer R, Pieber TR. Short acting insulin analogues versus regular human insulin inpatients with diabetes mellitus. April 19, 2004. Update: September 21, 2005.
vii.Vardi M. Jacobson E, Nini A, Bitterman H. Intermediate acting versus long acting insulin for type 1 diabetes mellitus. July 16, 2008.
viii.Wagh N. Inhaled insulin in diabetes mellitus. August 28, 2002. Withdrawn November 12, 2008.
(See AHRQ. See NICE.)
Code of Federal Regulations (CFR) Title: 21, Volume 8, Part 812. Investigationsl Device Exemptions. Revised as of April 1, 2008. Online at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=812.3
Code of Federal Regulations (CFR) Title: 21, Volume 1, Part 50. Protection of Human Subjects. Revised as of April 1, 2008. Online at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?FR=50.50
Code of Federal Regulations (CFR) Title: 42, Parts 400, 405, and 426. Medicare Program: Review of national coverage determinations and local coverage determinations. Federal Register 2002 Aug 22;67(163):54534-54563. Online at: http://www.cms.gov/Medicare/Coverage/DeterminationProcess/downloads/FR08222002.pdf
Coghlan M, Leighton B. Glucokinase activators in diabetes management. Expert Opin. Investig. Drugs 2008;17(2):145-167.
Compher C, Frankenfield D. Roth-Yousey L. Review: Best practice methods to apply to measurement of resting metabolic rate in adults: A systematic review. Journal of the American Dietetic Association 2006 June;106(6):881-903.
Compilation of Social Security Laws, Part E-Miscellaneous Provisions, Definition of Services, Institutions, etc., Section 1861(s)(3). Online at: http://www.ssa.gov/OP_Home/SSact/title18/1861.htm
Conover CA, Rozovski SJ, Belur ER, Aoki TT, Ruderman NB. Ornithine decarboxylase activity in insulin-deficient states. Biochem J 1980 Nov 15;192(2):725-732.
Cooper K. Compliance Oversight Coordinator, Office of Human Research Protections. Eastern Virginia Medical School. Human Research Subject Protections Under Multiple Project Assurance M-1532 and Federal-wide Assurance (FWA) 3956. Jun 15, 2006. Online at:
http://www.hhs.gov/ohrp/detrm_letrs/YR06/jun06a.pdf
Correct Coding Initiative letter. Medically Unlikely Edit. July 30, 2009. Rosen/Parish. (Hardcopy available.) (See ACCP/ATS.)
Courtney CH, Atkinson AB, Ennis CN, Sheridan B, Bell PM. Comparison of the priming effects of pulsatile and continous insulin delivery on insulin action in man. Metabolism 2003;52(8):1050-1055.
Cutfield W. Editorial: Short and sweet: the perinatal origins of type 2 diabetes mellitus. Pediatric Diabetes 2004 September 27;5(3):113-116.
Dailey GE, Boden GH, Creech RH, Johnson DG, Gleason RE, Kennedy FP, et al. Effects of Pulsatile Intravenous Insulin Therapy (PIVIT) on the progression of diabetic nephropathy. Metabolism 2000 November;49(11):1491-1495.
DeFronzo RA. Lilly Lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988 Jun;37(6):667-687.
DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, and Felber JP. The effect of insulin on the disposal of intravenous glucose: Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981 Dec;30:1000-1007.
Del Prato S, Marchetti P, Bonadonna RC. Phasic insulin release and metabolic regulation in type 2 diabetes. Diabetes 2000 Feb;51(Suppl 1):S109-S116.
DeMeyts P, Shymko RM. Timing-dependent modulation of insulin mitogenic versus metabolic signaling. Novartis Foundation symposium 2000. 27:46-70.
Department of Health and Human Services Departmental Appeals Board (HHS-DAB). California Appeal of the National Heritage Insurance Company Policy for Outpatient Intravenous Insulin Therapy.
Department of Health and Human Services, U.S. Food and Drug Administration. Regulatory Hearing on the proposal to disqualify Eugen O. Grecu, M.D., Ph.D. from receiving investigational new drugs: Summary decision of the Presiding Officer. Online at:
http://www.fda.gov/downloads/RegulatoryInformation/FOI/ElectronicReadingRoom/UCM143910.pdf
Department of the Army. Pulsatile Intravenous Insulin Therapy (PIVIT). (Personal communication with Dr. Robert Vigersky, Walter Reed, March 2009.)
Department of the Navy. Pulsatile Intravenous Insulin Therapy (PIVIT). (Personal communications with Dr. Patrick Clyde, National Naval Medical Center, Bethesda, Maryland, March 2009.)
DePergola G, Pannacciulli N, Minenna A, Martina R, Cannito F, Giorgino R. Fuel metabolism in adult individuals with a wide range of body mass index: Effect of a family history of type 2 diabetes. Diab Nutr Metab 2003 Feb;16(1):41-47.
DeSantis A, Schmeltz L, Schmidt K, O’Shea-Mahler E, Rhee C, Wells A. et al. Inpatient management of hyperglycemia: the Northwestern experience. Endocrine Practice 2006. Sep-Oct;12(5):491-505.
Devaraj S, Cheung AT, Jialal I, Griffen SC, Nguyen D, Glasser N, et al. Evidence of increased inflammation and microcirculatory abnormalities inpatients with type 1 diabetes and their role in microvascular complications. Diabetes 2007 Nov;56(11):2790-2796.
Dezaki K, Sone H, Koizumi M, Nakata M, Kakei M, Nagai H, et al. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes 2006 Dec;55(12):3486-3493.
Diabetes Control and Complications Trial (DCCT) Research Group. Implementation treatment protocols in the diabetes control and complications trial. Diabetes Care 1995. 18:361-376.
Diabetes Control and Complications Trial (DCCT) Research Group. Lifetime benefits and costs of intensive therapy as practiced in the diabetes control and complications trial. JAMA 1996. 276(17):1409-1415.
Diabetes Control and Complications Trial (DCCT) Research Group, Resource utilization and costs of care in the diabetes control and complications trial. Diabetes Care 1995. 18:1468-1478.
Diabetes Control and Complications Trial (DCCT) Research Group, The absence of a glycemic threshold for the development of long-term complications: the perspective of the diabetes control and complications trial. Diabetes 1996. 45:1289-1298.
Diabetes Control and Complications Trial (DCCT) Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. NEJM 1993. 329(14):977-986.
Diabetes Network of Researchers, Physicians and Healthcare Professionals. (See VitalCare.) (See Strategic Partners.) Online at: http://www.diabetes.net/
Diabetex International Corporation Securities Exchange Commission (SEC). (See SEC. See ADRI.) Online at: http://www.secinfo.com/d11Eeb.4f89d.b.htm
Diamond E. Developing a Cardiopulmonary Exercise Testing laboratory. American College of Chest Physicians (ACCP). Topics in Practice Management. Chest 2007 December 6;132:2000-2007. Online at: www.chestjournal.org
Dickerson R. Swiggart C, Morgan L, Maish G, Croce M, Minard G, et al. Safety and efficacy of a graduated intravenous insulin infusion protocol in critically ill trauma patients receiving specialized nutritional support. Nutrition 2008 June;24:536-545.
Dierschke DJ, Bhattacharya AN, Atkinson LE, Knobil E. Chichoral oscillations of plasma LH levels in the ovariectomized rhesus monkey. Endocrinology 1970 Nov;87(5):850-853.
Docherty K. Growth and development of the islet of Langerhans: implications for the treatment of diabetes mellitus. Curr Opin Pharmacol. 2001 Dec;1(6):641-650.
Donovan RM, Goldstein E, Kim Y, Lippert W, Kailath E, Aoki TT, et al. A computer-assisted image-analysis system for analyzing polymorphonuclear leukocyte chemotaxis inpatients with diabetes mellitus. J Infec Dis 1987 Apr;155(4):737-741.
Dortch MJ, Mowery NT, Ozdas A, Dossett L, Cao H, Collier B, et al. Original Communications. A computerized insulin infusion titration protocol improves glucose control with less hypoglycemia compared to a manual titration protocol in a trauma intensive care unit. JPEN. J Parenter Enteral Nutr 2008 Jan-Feb;32(1):18-27.
Duckworth W, Abraira C, Moritz T, Reda D, Emanuel N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. NEJM 2009 January 8;360(2):129-139.
Duke University Medical Center Library “Evidence-based Clinical Practice Resources" at http://www.mclibrary.duke.edu/subject/ebm/ebmpyramid.html.
Ebiner JR, Acheson KJ, Doerner A, Maeder E, Arnaud MJ, Jequier E, et al. Comparison of carbohydrate utilization in man using indirect calorimetry and mass spectrometry after an oral load of 100 g naturally-labelled [13C] glucose. Br J Nutr 1979;41:419-429.
Endocrine Society
(The Endocrine Society does not have any guidelines that address use of this therapeutic modality or the use of calorimetry in diabetic patients.)
The Endocrine society has a single guideline regarding diabetes.
Primary Prevention of Cardiovascular Disease and Type 2 Diabetes inpatients at Metabolic risk: An Endocrine Society Clinical Practice Guideline-2007. JL Rosenzweig, E Ferrannini, SM Grundy, SM Haffner, RJ Heine, ES Horton, R Kawamori.
Engdahl JH, Veidhuis JD, Farrell PA. Altered pulsatile insulin secretion associated with endurance training. J Appl Physiol 1995 Dec;79(6):1977-1985.
Fallon Community Health Plan. Insulin pumps and insulin pump supplies. Number: 200401-0005. Exclusion from coverage since it is considered experimental and investigational or unproven. Chronic Intermittent Intravenous Insulin Therapy (CIIIT) also referred to as Metabolic Activation Therapy (MAT), or Pulsatile Intravenous Insulin Therapy (PIVIT). Online at: http://www.fchp.org/NR/rdonlyres/262068FD-5BA5-4E9E-999E-6A470B8DCB6E/0/insulinpumps.pdf
Federal Trade Commission: Protecting America’s Consumers: United States of America Federal Trade Commission Washington, D.C. 1996. Online at: http://www.ftc.gov/bc/adops/ama.shtm
Felber JP, Magnenat G, Casthélaz M, Gesser CA, Müller-Hess R, de Kalbermatten N, et al. Carboyhydrate and lipid oxidation in normal and diabetic subjects. Diabetes 1977 July;26(7): 693-699.
Felber JP, Meyer HU, Curchod B, Iselin HU, Rousselle J, Maeder E, et al. Glucose storage and oxidation in different degrees of human obesity measured by continuous indirect calorimetry. Diabetologia 1981b Spring. 20:39-44.
Felber JP, Meyer HU, Cuchod B, Maeder E, Pahud P, Jéqueir E. Effect of a 3-day fast on glucose storage and oxidation in obese hyperinsulinemic diabetics. Metabolism 1981a February;30(2):184-188.
Ferrannini E. The theoretical bases of indirect calorimetry: A review. Metabolism 1988 March;37(3):287-301.
Feurer I, Mullen JL. Bedside measurement of resting energy expenditure and respiratory quotient via indirect calorimetry. Nutr Clin Pract 1986. 1:43-49.
First Coast Service Options (FCSO). Article for NCSVCS: The List of Medicare on covered Services-99199 Pulsatile Intraveneous Insulin Therapy (PIVIT) – Article Clarification (A48110) July 30, 2008. http://www.cms.gov/MCD/viewarticle.asp?article_id=48110&article_version=2&show=all
First Coast Service Options (FCSO) LCD for "List of Medicare Noncovered Services" (L5780) lists Pulsatile Intravenous Insulin Therapy, aka Metabolic Activation Therapy (99199) as a service that is never covered by the Medicare program. http://www.cms.gov/MCD/m_d.asp?id=5780&ver=54&cid=00590
Food and Drug Administration (FDA). Advisory Committee Meeting Transcript for Glucose Monitors. 2001. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfAdvisory/details.cfm?mtg=260
Food and Drug Administration (FDA). Alerts for Glucose Meters. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/GlucoseTestingDevices/ucm162016.htm and http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm049051.htm and http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/TipsandArticlesonDeviceSafety/ucm109371.htm and http://www.accessible-devices.com/glucose.html and http://www.consumeraffairs.com/news04/2005/fda_glucose_meters.html and http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ucm155099.htm and http://www.fda.gov/MedicalDevices/ProductsandMediclProcedures/InVitroDiagnostics/ucm.123672.htm
Food and Drug Administration (FDA). Apidra Label Use. Online at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021629s001,002lbl.pdf
Food and Drug Administration (FDA). Center for Devices and Radiological Health. Review Criteria Assessment of Portable Blood Glucose Monitoring in Vitro Diagnostic Devices Using Glucose Oxidase, Dehydrogenase or Hexokinase Methodology. Draft Document. Online at: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm094134.htm
Food and Drug Administration (FDA). Center for Devices and Radiological Health. Review of HemoCue Device. BK 060048 letter. Online at: http://www.consumeraffairs.com/news04/2005/fda_glucose_meters.html
Food and Drug Administration (FDA). Center for Devices and Radiological Health. Review of i-Stat Device. Online at: http://google2.fda.gov/search?q=i-STAT&client=FDAgov&site=FDAgov&lr=&proxystylesheet=FDAgov&output=xml_no_dtd&getfields=*
Food and Drug Administration (FDA). Center for Devices and Radiological Health. Title 21 – Food and Drugs, Chapter I – Food and Drug Administration Department of Health and Human Services, Subchapter H – Medical Devices, Part 868, Anesthesiology Devices. Online at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=868
Food and Drug Administration (FDA). Dear Doctor Letter. Exubra. 2008. DOC, Drugs.com. Online at: http://www.fda.gov/downloads/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm126610.pdf
Food and Drug Administration (FDA). Investigational New Drug (IND) or Device Exemption (IDE) Process (CBER): Vaccines, Blood and Biologics. Online at: http://www.fda.gov/BiologicsBloodVaccines/DevelopmentApprovalProcess/InvestigationalNewDrugINDorDeviceExemptionIDEProcess/default.htm
Food and Drug Administration (FDA). Medical Devices: Guidance on the Content of Premarket Notification [510(k)] Submissions for External Infusion Pumps. Online at: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm081337.htm
Food and Drug Administration (FDA). Meter Test Reagent Warnings. Online at: http://www.fda.gov/BiologicsBlood Vaccines/SafetyAvailability/ucm155099.htm
Food and Drug Administration (FDA). Medical Devices: Products and medical procedures. Online at: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm123672.htm
Food and Drug Administration (FDA). Meter Review Criteria. Online at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfAdvisory/details.cfm?mtg=260
Food and Drug Administration (FDA). Regular human insulin (Novolin R in 2005) Label Use. Online at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/019938s048lbl.pdf
Food and Drug Administration (FDA). Regulatory Information: "Off-Label" and Investigational Use of Marketed Drugs, Biologics, and Medical Devices – Information Sheet. Online at: http://www.fda.gov/RegulatoryInformation/Guidances/ucm126486.htm
Food and Drug Administration (FDA). Request COSMED SRl, Italy. COSMAD, fitmate Review of portable metabolic cart and summary including indications for use. 2007. Online at: http://www.accessdata.fda.gov/cdrh_docs/pdf7/K071533.pdf
Foss MC, Aoki TT. Effects of glucose loads of 50 and 100 g on carbohydrate and lipid oxidation in normal human subjects. Braz j Med Biol Res 1988;21(4):773-779.
Foss MC, Aoki TT. Restoration of fuel homeostasis in IDDM patients during pregnancy by an open-loop insulin infusion system. Diabetes Care 1993 January;16(1):103-109.
Foss MC, Cunningham LN, Aoki TT. Hormone-fuel metabolism during exercise of insulin-dependent diabetic patients treated with an artificial B-cell unit. Acta Diabetol Lat 1989 Jul-Sep;26(3):185-194.
Foss MC, Viachokosta FV, Cunningham LN, Aoki TT. Restoration of glucose homeostasis in insulin-dependent diabetic subjects: An inducible process. Diabetes 1982. 31(1):46-52.
Foss MC, Flachokosta FV, Aoki TT. Carbohydrate, lipid and amino acid metabolism of insulin-dependent diabetic patients regulated by an artificial beta-cell unit. Diabetes Res 1989 May;11(1):1-8.
Foss MC, Von-Oeyen P, Vlachokosta FV, Aoki TT. Fuel oxidation by insulin-dependent diabetics during late pregnancy in response to an oral glucose load. Braz J Med Biol Res 1992;25(2):135-144.
Fryback DG, Thornbury JR. The efficacy of diagnostic imaging. Med Decis Making 1991;11(2):88-94.
Furnary AP, Zerr KJ, Grunkemeier GI, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sterna wound infestion in diabetic patients after cardiac surgical procedures [with discussion]. Ann Thorac Surg 1999. 67:352-362.
Furnary AP, Gao G, Grunhemeir GL, Wa Y, Zeor KJ, Borkin So, et al. Continuous insulin infusion mortality inpatients with diabetews underging coronary artery bypass grafting. J Thorac Cardiovasc Surg 2003 May;125(5):1007-1021.
Fujii TK, Phillips BJ. Quick review: The metabolic cart. Internet J Intern Med. 2003. 3(2). Online at:http://www.ispub.com/journal/the_internet_journal_of_internal_medicine/volume_3_number_2_25/article_printable/quick_review_the_metabolic_cart.html
Fujita Y, Herron AL, Seltzer HS. Confirmation of impaired early insulin response to glycaemic stimulus in non-obese mild diabetics. Diabetes 1975 January;24:17-27.
Gaines AR, Pierce LR, Bernhardt PA. Fatal Iatrogenic Hypoglycemia: Falsely elevated blood glucose readings with a point-of-care meter due to a maltose-containing intravenous immune globulin product. Food and Drug Administration 2005. Online at: http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ucm155099.htm
Galvin P, Ward G, Walters J, Pestell R, Koschmann M, Vaag A, et al. A simple method for quantitation of insulin sensitivity and insulin release from an intravenous glucose tolerance test. Diabetic Medicine 1992. 9:921-928.
Gandhi G, Murad M, Flynn D, Erwin P, Cavalcante A, Bay NH, et al. Effect of perioperative insulin infusion on surgical morbidity and mortality: Systematic review and meta-analysis of randomized trials. Mayo Clin Proc 2008 April;83(4):418-430.
Gilbert Pump Patents. Strategic Partners: Bionica. VCTE Strategic partners. November 20, 2007. Online at: http://docs.google.com/gview?a=v&q=cache:6PpfL80xit4J:www.vitalcaretechnology.com/Pdf/Strategic%20Partners%20feb2908.pdf+Gilbert+Pump+Patents&hl=en&gl=us
Gill G, Williams G. Long-term intermittent intravenous therapy and type 1 diabetes. Lancet 1993 Oct 23;342(8878):1056-1057.
Gill G, Williams Gareth, Lamb WH (Editorial). Long-term intermittent intravenous therapy and type 1 diabetes mellitus. Lancet 1993 Oct 23;342:1056-1057.
Golay A, Munger R, Assimacopoulos-Jeannet F, Bobbioni-Harsch E, Habicht F, Felber J. Progressive defect of insulin action on glycogen synthase in obesity and diabetes. Metabolism 2002 May;51(5):549-553.
Goodman MN, Ruderman NB, Aoki TT. Glucose and amino acid metabolism in perfused skeletal muscle. Effect of dichloroacetate. Diabetes 1978 Nov;27(11):1065-1074.
Goodner CJ, Hom FG, Koerker DJ. Hepatic glucose production oscillates in synchrony with the islet secretory cycle in fasting rhesus monkeys. Science 1982 Mar 5;215(4537):1257-1260.
Goodner CJ, Walike BC, Koerker DJ, Ensinck JW, Brown AC, Chideckel EW, et al. Insulin glucagon, and glucose exhibit synchronous, sustained oscillations in fasting monkeys. Science 1977 Jan 14;195(4274):177-179.
Granato L, Brandes A, Bruni C, Greco AV, Mingrone G. V02, VC02 and RQ in a respiratory chamber: accurae estimation based on a new mathematical model using the Kalman-Bucy method. J Appl Physiol 2004. 96:1045-1054.
Grecu. Food and Drug Administration (FDA) Sanction.
Gredal C, Rosenfalck AM, Dejgaard A, Hilsted J. Impaired first-phase insulin response predicts postprandial blood glucose increment in patients with recently diagnosed type 2 diabetes. Scand J Clin Lab Invesst 2007. 67(3):327-336.
Grimsby J, Sarabu R, Corbett W, Haynes N, Bizzarro F, Coffey J, et al. Allosteric activators of glucokinase: Potential role in diabetes therapy. Science 2003 July 18; 301:370-373.
Grissinger M. Reducing medication errors associated with intravenous insulin infusions. Pharmacy and Therapeutics 2003;28(10):628.
Gromada J, Franklin I, Wolheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007 Feb;28(1):84-116.
Grubert JM, Lautz M, Lacy DB, Moore MC, Farmer B, Penaloza A, et al. Impact of continuous and pulsatile insulin delivery on net hepatic glucose update. Am J Physiol Endocrinol Metab 2005. 289:E232-E240.
Guimarães GV, Carvalho VO, Bocchi EA. Clinical Science: Reproducibility of the self-controlled six-minute walking test in heart failure patients. Clinics 2008. 63(2):201-206.
Gulan M, Perlman K, Albisser MA, Pyper J, Zinman B. Controlled crossover study of subcutaneous and intravenous insulin infusion in type I diabetes. Diabetes Care 1987 July-August;10(4):453-460.
Gunn JM, Taylor CB. Relationships between concentration of hepatic intermediary metabolites and induction of the key glycolytic enzymes in vivo. Biochem J 1973 Nov;136(3):455-465.
Hagedorn H, Jensen B, Krarup N, Wodstrup I. Protamine insulinate. J. Am. Med. Assoc 1936 Jan 18;106:177-180.
Hammond GS, Aoki TT. Measurement of health status in diabetic patients. Diabetes impact measurement scales. Diabetes Care 1992 Apr;15(4):469-477.
Hariharan N, Farrelly D, Hagan D, Hillyer D, Arbenny C, Sabrah T, et al. Expression of human hepatic glucokinase in transgenic mice liver results in decreased glucose levels and reduced body weight. Diabetes 1997 January;46:11-16.
Harrower AD. Treatment of diabetic ketoacidosis by direct addition of insulin to intravenous infusion. A comparison of "high dose" and "low dose" techniques. Br J Clin Pract 1979 Mar;33(3):85-86.
HAYES Alert Technology Assessment Brief. "Chronic Intermittent Intravenous Insulin Therapy for Type 1 DM". June 2006.
HAYES Medical Technology Directory. "Chronic Intermittent Intravenous Insulin Therapy (CIIIT) for Type 1 Diabetes Mellitus". July 14, 2006. Updated August 15, 2007 and September 29, 2008. (Personal communication with Pat Williamson on May 21, 2009.)
Heard CR, Henry PA. Glucose tolerance and insulin sensitivity. Clin Sci 1969. 37:37-44.
Heinemann L. Chronic intermittent intravenous insulin therapy: Really a new therapeutic option? Diabetes Technology and Therapeutics 2001. 3(1):125-127.
Heinemann L. Comment regarding: Pulsatile Insulin Therapy. Profil Institute for Metabolic Resarch GmbH. Neuss, Germany. June 15, 2009.
Heinemann L, Sonnenberg GE, Hohmann A, Ritzenhoff A, Berger M, Benn J, et al. Pulsatile insulin infusion and glucose-homeostasis in well-controlled type 1 (insulin-dependent) diabetic patients. J Internal Medicine 1989. 226(5):325-330.
Heller S, Kozlovski P, Kurtzhals P. Insulin’s 85th Anniversary-An enduring medical miracle. Diabetes Res Clin Pract 2007 Nov;78(2):149-158.
Hellman R. A systems approach to reducing errors in insulin therapy in the inpatient setting. Endocr Pract 2004 Mar-Apr;10 Suppl (2):100-8.
Henney JE. FDA Commissioner. Letter: Affirming and adopting the Food and Drug Administration, Summary Decision of the Presiding Officer, dated March 25, 1999. Online at: http://www.fda.gov/downloads/RegulatoryInformation/FOI/Electronic Reading Room/UCM143923.pdf; and http://www.fda.gov/downloads/RegulatoryInformation/FOI/Electronic Reading Room/UCM143923.pdf
Henquin JC, Boitard C, Efendie S, Ferrannini E, Steiner DF, Cerasi E. Editorial. Insulin secretion: Movement at all levels. Diabetes 2002 Feb;51(Suppl 1):S1-S2.
Henry CJK. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutrition 2005. 87(7A):1133-1152. Online at: http://journals.cambridge.org/download.php?file=/PHN/PHN8_7a/S1368980005001394a.pdf&code=fcaf1352a42a9f4d892e95a86818a91c
Hills, CE, Brunskill NJ. Intracellular Signalling by C-peptide. Experimental Diabetes Research 2008;2008:635158-635166.
Hollander-Roderiguez JC, Calvert JF. Hyperkalemia. American Family Physiscian 2006 January 15;73(2):283-290.
Hollingdal M, Juhl CB, Pincus SM, Sturis J, Veldhuis JD, Polonsky KS, et al. Failure of physiological plasma glucose excursions to entrain high-frequency pulsatile insulin secretion in type 2 diabetes. Diabetes 2000. 49:1334-1340.
Holstein A, Plaschke A, Egberts E. Clinical characterization of severe hypoglycaemia—a prospective population-based study. Exp Clin Endocrinol Diabetes 2003 Sept;111:364-369.
[Johns] Hopkins Bayview Medical Center. General Clinical Research Center: Metabolic Cart Procedure. Policy No: 159, April 10, 2003. (See Johns.) Online at: http://jhbgcrc.jhu.edu/PolicyProcedure/Metabolic Cart Procedure 20030410.pdf
Home PD, Alberti KG. The new insulins. Their characteristics and clinical indications. Drugs 1982 Nov;24(5):401-413.
Hou JC, Min L, Pessin JE. Insulin granule biogenesis, trafficking and exocytosis. Chapter Sixteen. Vitamins and Hormones. Elsevier Inc., Amsterdam, The Netherlands. 2009;80:473-506.
Hovorka R. Continuous glucose monitoring and closed-loop systems. Diabet Med 2006 Jan;23(1):1-12.
Hovorka R. The future of continuous glucose monitoring: Closed Loop. Current Diabetes Reviews 2008. 4(3):269-279.
Huang ES, Basu A, O’Grady MJ, Capretta JC. Using clinical information to project federal health care spending. Health Affairs – Web Exclusive 2009 September 1:w978- w-990. Online at: http://content.healthaffairs.org/cgi/content/full/hlthaff.28.5.w978/DC1
Hunter SJ, Atkinson AB, Ennis CN, Sheridan B, Bell PM. Association between insulin secretory pulse frequency and peripheral insulin action in NIDDM and normal subjects. Diabetes 1996 May;45(5):683-686.
Iannuzzi A, Licenziati MR, Acampora C, Salvatore V, Marco DD, Mayer MC, et al. Preclinical changes in the mechanical properties of abdominal aorta in obese children. Metabolism 2004. 53(9):1243-1246.
Igawa S, Sakamaki M, Miyazaki M. Examination of the reliability of the portable calorimeter. Clinical and Experimental Pharmacology and Physiology 2002. 29;S13-S15.
Inside Wall Street Report: for Tuesday April 22nd, 2008. VitalCare Announces FDA approval of infusion device used for iCAT therapy. (See VitalCare) Online at: http://www.insidewallstreetreport.com/quotebox_VDTI_profile_042408.html
Jaffee RS, Aoki TT, Rohatsch PL, Disbrow EA, Fung DL. Predicting cardiac autonomic neuropathy in type 1 (insulin-dependent) diabetes mellitus. Clin Auton Res 1995 Jun;5(3):155-158.
Jarvis L. Lilly drops inhaled insulin: Move adds to growing list of failures for the technology. Chemical and Engineering News. 2008 March 17;86(11):9. Online at: http://pubs.acs.org/cen/news/86/i11/8611notw2.html
Jensen K, Jørgensen S, Johnsen L. A metabolic cart for measurement of oxygen uptake during human exercise using inspiratory flow rate. Eur J Appl Physiol 2002;87:202-206.
Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 2008 Dec;295(6):E1287-1297.
Jéquier E, Felber J. Indirect calorimetry. Bailliére’s Clin Endocrin and Metab 1987 Nov;1(4):911-935.
Johns Hopkins Bayview Medical Center. General Clinical Research Center: Metabolic Cart Procedure. Policy No: 159, April 10, 2003. (See Hopkins) Online at: http://jhbgcrc.jhu.edu/PolicyProcedure/Metabolic Cart Procedure 20030410.pdf
Joint Commission on Accreditation of Healthcare Organizations (JCAHO). Joint commission IDs five high-alert meds. ED Manag 2000 Feb;12(2):21-22.
Juhl CB, Porksen N, Hollingdal M, Sturis J, Pincus S, Veldhuis JD, et al. Repagliride acutely amplifies pulsatile insulin secretion by augmentation of burst mass with no effect on burst frequency. Diabetes Care 2000;23(5):675-681.
Juhl CB, Porksen N, Pincus SM, Hansen ÅP, Veldhuis JD, Schmitz O. Acute and short-ter administration of a sulfonylurea (Gliclazide) increases pulsatile insulin secretion in type 2 diabetes. Diabetes 2001;50:1778-1784.
Juhl CB, Schmitz O, Pincus S, Holst JJ, Veldhuis J, Porksen N. Short-term treatment with GLP-1 increases pulsatile insulin secretion in Type II diabetes with no effect on orderliness. Diabetologia 2000;43:583-588.
Juhl CB, Sturis J, Hollingdal M, Schmitz O. Acute insulin responses to intravenous glucose and GLP-1 are independent of preceding high-frequency insulin pulse-defects induced by glucose entrainment in Healthy Humans. Horm Metab Res. 2005;37:40-44.
Juvenile Diabetes Research Foundation (JDRF)
(The JDRF does not have clinical practice guidelines.)
Kanji S, Singh A, Tierney M, Meggison H, McIntyre L, Herbert PC. Standardization of intravenous insulin therapy improves the efficiency and safety of blood glucose control in critically ill adults. Intensive Care Med 2004 May;30(5):804-810.
Keegan A. Weak sales lead to Exubera’s market withdrawal. ADA DOC News. December 2007. Online at: http://docnews.diabetesjournals.org/content/4/12/5.1.full
Kerner W, Brückel J, Zier H, Arias P, Thun C, Moncayo R, et al. Similar effects of pulsatile and constant intravenous insulin delivery. Diabetes Res Clin Pract 1988 Apr 6;4(4):269-274.
Khafagy E, Morishita M, Onuki Y, Takayama K. Current challenges in non-invasive insulin delivery systems:A comparative review. Adv Drug Delivery Rev 2007 August 16;59:1521-1546.
Kitabchi AE, Umpierrez GE, Murphy MB, et al. (American Diabetes Association). Hyperglycemic crises inpatients with diabetes mellitus. Diabetes Care 2003;26 Suppl (1):S109-S117.
Knapke CM, Owens JP, Mirtallo JM. Management of glucose abnormalities inpatients receiving total parenteral nutrition. Clin Pharm 1989;8:136-144.
Koch B. Selected topics of hypoglycemia care. Canadian Family Physician 2006 April;52:466-471.
Kocoglu H, Goksu S, Pence S, Soykan B, Kocak T, Yilmaz N. Insulin dose versus rate of potassium decrease in the treatment of hyperkalemia with IV insulin during extracorporeal circulation: an observational study. Current Therapeeutic Research 2002;63(9):549-555.
Koerker DJ, Goodner CJ, Hansen BW, Brown AC, Rubenstein AH. Synchronous, sustained oscillation of C-peptide and insulin in the plasma of fasting monkeys. Endocrinology 1978 May;102(5):1649-1652.
Koopmans AJ, Sips HC, Krans HM, Raddar JK. Pulsatile intravenous insulin replacement in streptozotocin-diabetic rats is more efficient than continous delivery: effects on glycaemic control insulin-mediated glucose metabolism and lipolysis. Diabetologia 1996 Apr;39(4):391-400.
Korp W, Levett RE. Experiences with monocomponent insulin treatment. Wien Klin Wochenschr 1973;85(18):326-330.
Kost GJ, Vu HT, Lee JH, Bourgeois P, Kiechie FL, Martin C, et al. Multicenter study of oxygen-insensitive handheld glucose point-of-care testing in critical care/hospital/ambulatory patients in the united States and Canada. Crit Car Med 1998 Mar;26(3):581-590.
Kumai M, Tamai H, Fujii S, Nakagawa T, Aoki TT. Glucagon secretion in anorexia nervosa. Am J Clin Nutr 1988 Feb;47(2):239-242.
Ladisch MR, Kohlmann KL. Recombinant human insulin. Biotechnol. Prog 1992;8(6):469-478.
Laedtke T, Kjems L, Porksen N, Schmitz O, Velduis J, Kao PC, et al. Overnight inhibition of insulin secretion restores pulsatility and proinsulin/insulin ratio in type 2 diabetes. Am J Physiol Endocrinol Metab 2000;279:E520-E528.
Lang DA, Matthews DR, Peto J, Turner RC. Cyclic oscillations of basal plasma glucose and insulin concentrations in human beings. N Engl J Med 1979 Nov8;301(19):1023-1027.
Lang DA, Matthews DR, Burnett M, Ward GM, Turner RC. Pulsatile, synchronous basal insulin and glucagon secretion in man. Diabetes 1982 Jan;31(1):22-26.
Lang DA, Matthews DR, Burnett M, Turner RC. Brief, irregular oscillations of basal plasma insulin and glucose concentrations in diabetic man. Diabetes 1981;30:435-439.
Lazar HL, Chipkin S, Phillippides G, Bao Y, Apstein C. Glucose-insulin-potassium solutions improve outcomes in diabetics who have coronary artery operations. Ann Thorac Surg 2000;70:145-150.
Larsen T, Jørgensen JO, Jakobsen G, Hansen BL, Christiansen JS. Continuous infusion versus daily injections of growth hormone (GH) for 4 weeks in GH-deficient patients. J Clin Endocrinol Metab 1995;80(8):2410-2418.
Laursen T, Jørgensen JO, Jakobsen G, Hansen BL, Christiansen JS. Continuous infusion versus daily injections of growth hormone (GH) for 4 weeks in GH-deficient patients. J of Clin endocrinology and Metabolism.1995;80(8):2410-2418.
Lavernia F. Treating hyperglycemia and diabetes with insulin therapy: transition from inpatient to outpatient care. J Med 2008 September 17;10(9):216. Online at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=docline&pubmedid-19008977.
Lefebvre PJ, Paolisso G, Scheen AJ, Henquin JC. Review. Pulsatility of insulin and glucagon release physiological significance and pharmacological implications. Diabetologia 1987 July;30(7):443-452.
Letter outlining the sale of VitalCare Technology enterprises (SELLER) to Modern Technology Corp (BUYER). July 4, 2007. Online at: http://www.secinfo.com/d1Z7X7.u7e.d.htm
Levy-Marchal C, Albisser AM, Zinman B. Overnight metabolic control with pulsed intermittent versus continuous subcutaneous insulin infusion. Diabetes Care 1983;6:356-360.
Lew’s Assessment of the statistical design of three studies on hepatic activation. (Submitted April 20, 2009 with public comment via mail from Michael Bradley, Clinic Manager, Aoki Diabetes Research Institute.)
Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. NEJM 1993; 329:1456-1462.
Lien L, Spratt S, Woods Z, Osborne K, Feinglos M. Optimizing hospital use of intravenous insulin therapy: improved management of hyperglycemia and error reduction with a new nomogram. Endocrine Practice 2005 Jul-Aug;11(4):240-253.
Logan-Darrough M. Pulsatile I.V. Insulin Therapy for severely out of control diabetes. J Intravenous Nursing 1995;18(3):124-128.
Luksch A, Polak K, Matulla B, Dallinger S, Kapiotis S, Rainer G, et al. Glucose and insulin exert additive ocular and renal vasodilator effects on healthy humans. Diabetologia 2001;44:95-103.
Maffeis C, Schutz Y, Chini L, Grezzani A, Piccoli R, Tató L. Effects of dinner composition on postprandial macronutrient. Obesity Research 2004 July;12(7):1128-1135.
Malmberg K (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction [DIGAMI] Study Group). Prospective randomized study of intensive insulin treatment on long term survival after acute myocardial infarction inpatients with diabetes mellitus. BMJ 1997 May;314(7093):1512-1515.
Mansell PI, Macdonald IA. Reappraisal of the Weir equation for calculation of metabolic rate. Am J Physiol Regulatory Integrative Comp Physiol 1990 Jun;258(6 Pt 2):1347-1354.
Mari A, Camastra S, Toschi E, Giancaterini A, Gastaldelli A, Mingrone G, et al. A model for glucose control of insulin secretion during 24 h of free living. Diabetes.2001 Feb;50(Supple 1):164-168.
Marliss EB, Ohman JL Jr, Aoki TT, Kozak GP. Altered redox state obscuring ketoacidosis in diabetic patients with lactic acidosis. NEJM 1970 Oct 29;283(18):978-980.
Martens GA, Pipeleers D. Glucose, regulator of survival and phenotype of pancreatic beta cells. Chapter Seventeen. Vitamins and Hormones. Academic Press. Elsevier Inc., Amsterdam, The Netherlands. 2009;80:507-539.
MAT Protocol. (Submitted April 20, 2009 with public comment via mail from Michael Bradley, Clinic Manager, Aoki Diabetes Research Institute.) (Hardcopy available.) (See ADRI.)
Mateo F, Torres NV, Meléndez-Hevia E. Role of hexokinase in controlling the glucose metabolism flux: A study of its flux control coefficient in different tissues. Cellular and Molecular Biology 1989;35(1);33-37.
Matschinsky FM, Magnuson MA, Zelent D, Jetton TL, Doliba N, Yan Y, et al. The network of glucokinase-expressing cells in glucose homeostasis and the potential of glucokinase activators for diabetes therapy. Diabetes 2006 January;55:1-12.
Matthews DR. Physiological implications of pulsatile hormone secretion. Am NY Acad Sci 1991;618:28-37.
Matthews DR, Lang DA, Burnett MA, Turner RC. Control of pulsatile insulin secretion in man. Diabetologia 1983 Apr;24(4):231-237.
Matthews DR, Naylor BA, Jones RG, Ward GM, Turner RC. Pulsatile insulin has greater hypoglycemic effect. Diabetes 1983 Jul;32(7):617-621.
McCarren M. [Special Report] Make the most of your meter. Diabetic Living. Fall 2009:39-47.
McClave SA, Lowen CC, Kleber MJ, McConnell JW, Jung LY, Goldsmith LJ. Clinical use of the respiratory quotient obtained from indirect calorimetry. J Parent Enterol Nutr 2003 Jan/Feb;27(1):21-26.
McClave SA, Snider HL. Use of indirect calorimetry in clinical nutrition. Nutr Clin Pract: Official Publication of the American society for Parenteral and Enteral Nutrition 1992 Oct;7(5):207-221.
McGregor IS, Lee AM. Metabolic changes associated with ingestion of different macronutrients and different meal sizes in rats. Physiol Behav 1995 Feb;57(2):277-286.
Medicare Benefit Policy Manual, Chapter 15, Section 50.2. Determining self-administration of drug or biological. Internet Only Manual 100-02. Online at: http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/bp102c15.pdf
Medicare Contractor Local Coverage Determinations Process. Online at: http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/pim83c13.pdf
Medicare Decision Memo for Clinical Trial Policy (CAG-00071R). Online at: http://www.cms.gov/mcd/viewdecisionmemo.asp?id=186
Medicare Evidence Development and Coverage Advisory Committee (MedCAC). Glycemic Control. August 2006. Online at: http://www.cms.gov/mcd/viewmcac.asp?where=index&mid=36
Medicare Guidance for the Public, Industry, and CMS Staff National Coverage Determinations with Data Collection as a Condition of Coverage: Coverage with Evidence Development: Document Issued on July 12, 2006. Online at: https://www.cms.gov/mcd/ncpc_view_document.asp?id=8
Medicare Local Coverage Determination (LCD) for Diabetes Mellitus Therapy using Hepatic Activation or PIVIT Therapy (L28252). Online at: http://www.cms.gov/mcd/viewlcd_pdf.asp?lcd_id=28252&lcd_version=10&contractor_id=174
Medicare. National Correct Coding Initiatives (NCCI) Edits. Online at: http://www.cms.gov/Medicare/Coding/NationalCorrectCodInitEd/index.html#TopOfPage
Medicare National Coverage Determination (NCD) for Closed-Loop Blood Glucose Control Device (CBGCD) (40.3). Online at: http://www.cms.gov/mcd/viewncd.asp?ncd_id=40.3&ncd_version=1&basket=ncd:40.3:1:Closed-Loop+Blood+Glucose+Control+Device+(CBGCD) >
Medicare National Coverage Determination (NCD) for Home Blood Glucose Monitors 40.2. Online at: http://www.cms.gov/mcd/viewncd.asp?ncd_id=40.2&ncd_version=2&basket=ncd:40.2:2:Home+Blood+Glucose+Monitors
Medicare National Coverage Determination (NCD) for Insulin Infusion Pump 280.14. Online at: http://www.cms.gov/mcd/viewdecisionmemo.asp?id=40
Medicare Part B systems Extract and Summary System (BESS) Procedure Summary File for Respiratory quotient CPTs=94681; 94690, 94621 in 2000-2007. Data pulled 2008.
Medicare Program Memorandum for Intermediaries/Carriers: AB-00-108. Glucose Monitoring. Change Request 1362. December 1, 2000. Online at: http://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/Downloads/AB00108.pdf
Medicare Medically Unlikely Edits (MUEs). Online at: http://www.cms.gov/Medicare/Coding/NationalCorrectCodInitEd/MUE.html
Meguro S, Funae O, Hosokawa K. Atsumi Y. Hypoglycemia detection rate differs among blood glucose monitoring sites. Diabetes Care 2005 March;28(3);708-709.
Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes 2005;(54):1649-1656.
Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes 2005;(54):1649-1656.
Meistas MT, Vlachokosta FV, Gleason RE, Arcangeli M, Aoki TT. Role of muscle in C02 production after oral glucose administration in man. Diabetes 1985 Oct;34(10):960-963.
Meneghini L. Review Article. Demonstrating strategies for initiation of insulin therapy: matching the right insulin to the right patient. Int J Clin Pract 2008 Aug;62(8):1255-1264.
Meneilly GS, Veldhuis JD, Elahi, D. Disruption of the pulsatile and entropic modes of insulin release during an unvarying glucose stimulus in elderly individuals. J Clin Endocrinol Metab 2009;84(6):1938-1943.
Metabolic Industries (MI). Online at: http://www.metabolicactivationtherapy.com/company.htm and http://www.metabolicactivationtherapy.com/news.htm and http://www.metabolicactivationtherapy.com/no-rights-to-mat.htm
Meyer HU, Curchoo B, Maeder E, Pahud P, Jéquer E, Felber JP Modifications of glucose storage and oxidation in nonobese diabetics, measured by continuous indirect calorimetry. Diabetes 1980 September;29:752-756.
Millis RM, Austin RE, Bond V, Faruque M, Goring KL, Hickey BM, et al. Effects of high-carbohydrate and high-fat dietary treatments on measures of heart rate variability and sympathovagal balance. Life Sciences 2009 Jul 17;85(3-4):141-145.
Mirbolooki, MR, Taylor GE, Knutzen VK, Scharp DW, Wilcourt R, Lakey JR. Pulsatile intravenous insulin therapy: The best practice to reverse diabetes complications? 2009. Article in Press. Online at: http://www.sciencedirect.com/science?_ob=MImg&_imagekey=B6WN2-4W99NT7-1-5&_cdi=6950&_user=10843&_orig=search&_coverDate=05/15/2009&_sk=999999999&view=c&wchp=dGLbVlz-zSkWA&md5=b44130a2e5340e4360cb0155e01dd80a&ie=/sdarticle.pdf
Moeri R, Golay A, Schutz Y, Temler E, Jequier E, Felber JP. Oxidative and nonoxidative glucose metabolism following graded doses of oral glucose in man. Diabete & Metabolism 1988;14:1-7.
Moore L. Novo Nordisk refocuses its activities within inhaled insulin and discontinues the development of AERx. Drugs.com. January 14, 2008. Online at: http://www.drugs.com/news/novo-nordisk-refocuses-activities-within-inhaled -insulin-discontinues-development-aerx-7555.html
Moore MC, Cherrington AD, Wasserman DH. Regulation of hepatic and peripheral glucose disposal. Regulation of hepatic glucose disposal by metabolites and hormones. Best Practice & Research Clinical Endocrinology & Metabolism 2003;17(3):343-364.Muller WA, Brennan MF, Tan MH, Aoaki TT. Studies of glucagon secretion in pancreatectomized patients. Diabetes1974 Jun;23(6):512-516.
Mulrow CD Lohr KN. Proof and Policy from Medical Research Evidence. J Health Policy Law 2001 Apr;26(2):249-266.
Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy. Diabetes Care 2008 Dec;32(12):1-11.
Nathan DM, Dunn FL, Bruch J, McKitrick C, Larkin M, Haggan C, et al. Posprandial insulin profiles with implantable pump therapy may explain decreased frequency of severe hypoglycemia, compared with intensive subcutaneous regimens, in insulin-dependent diabetes mellitus patients. Am J of Med 1996;100:412-417.
National Collaborating Center for Chronic Conditions. Type 1 diabetes in adults. National clinical guideline for diagnosis and management in primary and secondary care. London (UK): Royal College of Physicians. 2004. [382 references]:171. Online at: http://www.guideline.gov/summary/summary.aspx?doc_id=6249
National Heritage Insurance Company (NHIC) followed by Palmetto GBA. Noncoverage PIVIT Policy. Circa 2000. (See Appendix 14 NHIC-Palmetto Policy.) Online at: http://coverage.cms.fu.com/mcd_archive/search.asp?clickon=search; and http://www.cms.gov/mcd/viewlcd.asp?lcd_id=28252&lcd_version=10&=all
National Institute of Health/Food and Drug Administration Workshop. Closed-loop insulin infusion systems (Artificial pancreas). 2008. Online at: http://diabetes.niddk.nih.gov/about/dateline/fall08/1.htm; and http://videocast.nih.gov/launch.asp?14598 and http://videocast.nih.gov/launch.asp?14601
National Institute for Health and Clinical Excellence (NICE) External Technology Assessments
i.Simultaneous pancreas-kidney transplants in diabetic patients. January 2008. Updated January 2009.
ii.Pancreas after kidney transplantation in diabetic patients. June 2006. Updated July 2008.
iii.Needle-free insulin injection systems. September 18, 2006.
iv.Pancreas transplantation alone. Marcy 1999. Updated February 26, 2006.
v.Islet cell transplantation for the treatment of type 1 diabetes. August 2004. Updated August 2005.
vi.Guideline Development Group. Type 2 diabetes: The management of diabetes (update) NICE guideline CG66 Diabetes – type 2 (update): December 5, 2008 vs. May 2008.
Diabetes (type 2)-glitazones TA63. August 2003 (but replaced by CG66)
Diabetes (type 2)-ioglitazone TA21. March 2001 (but replaced by TA63)
Diabetes (type 2)-rosiglitazone TA9. August 2000 (but replaced by TA63)
vii.Aberdeen HTA Group Continuous subcutaneous insulin infusion for the treatment of diabetes (review) TA151. July 2008.
Southampton HTA, University of Southampton. The clinical effectiveness and cost effectiveness of insulin pump therapy TA57. April 2003.
viii.Aberdeen HTA Group. Inhaled insulin for the treatment of type 1 and type 2 diabetes TA113. Made obsolete: January 2008
Diabetes (types 1 and 2) - Patient education models. August 2003.
ix.School of Health and Related Research. University of Sheffield. The clinical effectiveness and cost effectiveness of long acting insulin analogues for diabetes TA53. December 2002.
(See AHRQ. See Cochrane.)
Nielsen S, Guo ZK, Albu JB, Klein S, O’Brien PC, Jensen MD. Energy expenditure, sex, and endogenous fuel availability in humans. J of Clinical Investigation 2003 April;111(7):981-988.
Nig LL, Coppack SW, Zhang L, Hockaday TD. Application of minimal models to measuring insulin sensitivity. Horm Metab Res 1990;24(Suppl):57-59.
Nordquist L, Johansson M. Proinsulin C-peptide: Friend or foe in the development of diabetes-associated complications? Vascular Health and Risk Management 2008;4(6):1283-1288.
Noridian A29261: Chronic Intermittent Intravenous Insulin Therapy or Pulsatile IV Insulin Therapy. Article clarifying noncoverage of MAT. Jurisdiction: IA. Online at: http://www.cms.gov/MCD/m_a.asp?id=29261&ver=2&cid=00826
Noridian A28853: Chronic Intermittent Intravenous Insulin Therapy or Pulsatile IV Insulin Therapy. Article clarifying noncoverage of MAT. Jurisdiction: AL, AS, Guam, HI, NV, OR, WA, Northern Mariana Islands. Online at: http://www.cms.gov/MCD/m_a.asp?id=28853&ver=3&cid=00821
Noridian A27633: Chronic Intermittent Intravenous Insulin Therapy or Pulsatile IV Insulin Therapy. Article clarifying noncoverage of MAT. Jurisdiction: CO. Online at: http://www.cms.gov/MCD/m_a.asp?id=27633&ver=4&cid=00820.
Normedex Company Overview and PIVIT Insurance. Online at: http://www.normedex.com/normedex/contact.html; and http://www.normedex.com/normedex/treatment.html; and http://www.normedex.com/normedex/faq.html; and http://www.normedex.com/normedex/insurance.html; and http://www.normedex.com/normedex/diabetes.html; and http://www.normedex.com/normedex/company.html
O’Doherty RM, Lehman DL, Télémaque-Potts S, Newgard CB. Metabolic impact of glucokinase overexpression in liver: Lowering of blood glucose in red rats is accompanied by hyperlipidemia. Diabetes 1999 October;48:2022- 2027.
ODS Health Plan, Inc. Insulin Pumps. Most current Revision: August 2008 and approved by Csaba Mera, MD on January 18, 2008. Online at: http://www.odscompanies.com/pdfs/med_criteria/InsulinPumps.pdf.
Office of the Inspector General Letter. Glucose Monitoring. 1999. Online at: http://oig.hhs.gov/publications/docs/semiannual/1999/99semif.pdf; and http://oig.hhs.gov/oei/reports/oei-05-99-00380.pdf
Ohman JL Jr, Marliss EB, Aoki TT, Munichoodappa CS, Khanna VV, Kozak GP. The cerebrospinal fluid in diabetic ketoacidosis. NEJM 1971 Feb11;284(6):283-290.
Olson KR. Poisoning and drug overdose. Hyperkalemia and insulin. The McGraw-Hill Companies, Inc. 2007.
O’Rahilly S, Hosker JP, Rudenski AS, Matthews DR, Burnett, MA, Turner RC. The glucose stimulus-response curve of the ß-cell in physically trained humans, assessed by hyperglycemic clamps. Metabolism 1988 October;37(10):919-923.
O’Rahilly S, Trembath RC, Patel P, Galton DJ, Turner RC, Wainscoat JS. Linkage analysis of the human insulin receptor gene in Type 2 (non-insulin-dependent) diabetic families and a family with maturity onset diabetes of the young. Diabetologia 1988;31:792-797.
O’Rahilly S, Turner RC. Early-onset type 2 diabetes vs maturity-onset diabetes of youth: evidence for the existence of two discrete diabetic syndromes. Diabetic Medicine 1988;5:224-229.
O’Rahilly S, Turner RC. Linkage analysis of the receptor gene and MODY. Diabetologia 1988;31:184-187.
O’Rahilly S, Turner RC, Matthews DR. Impaired pulsatile secretion of insulin in reality? NEJM 1988 May 12;318(19):1225-1230.
O’Rahilly S, Wainscoat JS, Turner RC. Type 2 (non-insulin-dependent) diabetes mellitus. New genetics for old nightmares. Diabetologia 1998;31:407-414.
Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care 2006 November;29(11):2528-2538.
Overgaard RV, Jelic K, Karlsson M, Henriksen JE, Madesen H. Mathematical beta cell model for insulin secretion following IVGTT and OGTT. Annals of Biomed Engineering 2006;34(8):1343-1354.
Oxford – United Health Care. Policy Number: DIABETIC 017.1 T3. Effective: March 2009. Online at:
https://www.oxhp.com/secure/policy/intermittent_iv_insulin_therapy_309.html
Paice BJ, Paterson KR, Onyanga-Omara F, Donnelly T, Gray JM, Lawson DH. Record linkage study of hypokalaemia in hospitalized patients. Postgrad Med J 1986 March;62(725):187-191.
Pal M. Recent advances in glucokinase activators for the treatment of type 2 diabetes. Drug Discovery Today 2009 August;14(15/16):784-792.
Palmetto GBA, MAC – Part B, Diabetes Mellitus Therapy Using Hepatic Activation or PIVIT Therapy (L28252). September 2, 2008. Online at: http://www.cms.gov/mcd/viewlcd.......
Paolisso G, Salvatore T, Sgambato S, Torella R, Varricchio M, D’Onofrio F. Metabolic effects of pulsatile insulin infusion in the elderly. Endocrinol 1990 July;123(1):19-23.
Paolisso G, Scheen AJ, Albert A, and Lefebvre PJ. Effects of pulsatile delivery of insulin and glucagon in humans. Am J Physiol Endocrinol Metab 1989 November;257(20):E686-E696.
Paolisso G. Scheen AJ, Verdin EM, Luyckx AS, Lefebvre PJ. Insulin oscillations per se do not affect glucose turnover parameters in normal man. J Clin Endocrinol Metab 1986 August;63(2):520-525.
Paolisso G, Scheen AJ, Guigliano D, Sgambato S, Albert, A. Varricchio M, et al. Pulsatile insulin delivery has greater metabolic effects than continuous hormone administration in Man: importance of pulse frequency. J of Clin Endocrinol and Metab 1991 March;72(3):607-615.
Paolisso G, Sgambato S, Gentile S, Memoli P, Guigliano D, Varricchio M. Advantageous metabolic effects of pulsatile insulin delivery in noninsulin-dependent diabetic patients. J Clin Endocrinol Metab 1988 November;67(5):1005-1010.
Paolisso G. Sgambato S, Guinta R, Varricchio M, D’Onofrio F. Pulsatile rather than continuous glucagon infusion leads to greater metabolic derangements in insulin-dependent diabetic subjects. Diabete Metab 1990 Jan-Feb;16(1):42-47.
Paolisso G, Sgambato S, Passariello N, Scheen, A, D’Onofrio F, Lefebvre PJ. Greater efficacy of pulsatile insulin in type 1 diabetics critically depends on plasma glucagon levels. Diabetes 1987;36(5):566-570.
Paolisso G, Sgambato S, Torella R, Varricchio M, Scheen A.D’Onofrio F, et al. Pulsatile insulin delivery is more efficient than continuous infusion in modulating islet cell function in normal subjects and patients with type 1 diabetes. J Clin Endocrinol and Metab.1988;66(6):1220-1226.
Paolisso G, Sgambato S, Varricchio M, Scheen AJ, D’Onofrio F, Lefebvre. Insulin effects on glucose kinetics in non-insulin-dependent diabetic patients with secondary failure to hypoglycaemic agents: Role of different modes and rates of delivery. European J Medicine 1992;1(5)261-267.
Parrish DM. Correct Coding Initiative letter. Medically Unlikely Edit. July 30, 2009. (See (ACCPand ATS and CCI-Rosen). (Hardcopy available.)
Park RH, Hansell DT, Davidson LE, Henderson G, Legge V, Gray GR. Applied Nutritional Investigation. Management of diabetic patients requiring nutritional support. Nutrition 1992 September/October;8(5):316-320.
Payne VA, Arden C, Lange AJ, Agius L. Contributions of glucokinase and phosphofructokinase-2/fructose bisphosphatase-2 to the elevated glycolysis in hepatocytes from Zucker fa/fa rats. Am J Physiol Regulatory Integrative Comp Physiol 2007 August;293:618-625.
Perera NJ, Twigg SM, Williams PF, Chua EL, Stewart PM, Yue DK. The Danger of using inappropriate point-of-care glucose meters inpatients on Icodextrin dialysis. Department of Clinical Biochemistry, Royal Prince Alfred hospital, Sydney, Australia. Diabetes Centre, Department of Endocrinology, Royal Prince Alfred Hospital, 2009. Sydney, Australia. Discipline of Medicine, University of Sydney, Sydney, Australia. P1-459.
Pezzarossa A, Taddei F, Cimicchi MC, Rossini E, Contini S, Bonora E, et al. Perioperative management of diabetic subjects: Subcutaneous versus intravenous insulin administration during glucose-potassium infusion. Diabetes Care 1988;11:52-58.
Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 1997;40:1286-1292.
Pickup S, Popescu M. Efficient design of pulses with trapezoidal magnitude and linear phase response profiles. Magnetic Resonance in Medicine: official journal of the Society of Magnetic Resonance in Medicine 1997 Jul;38(1):137-145.
Pickup JC, Roberts GA, Kehely AM, Pasapula C, Chusney GD, Mather HM. Higher serum sialic acid in women than in men with NIDDM: Possible relevance to increased cardiovascular risk in NIDDM women. Diabetes Care 1997 September;20(9):1496.
Pinkos A, Arreaza-Rubin G, Heetderks WJ, Irony I, Joffe HV, Schneider B. FDA’s proactive role in the development of an artificial pancreas for the treatment of diabetes mellitus. Drug Discovery today: Technologies. Elsevier 2007;4(1):25-28.
Piters KM, Kumar D, Pei E, Bessman AN. Comparison of continuous and intermittent intravenous insulin therapies for diabetic ketoacidosis. Diabetologia 1977;13:317-321.
Polak K,Dallinger, S, Polska, E, Findl O, Eichler HG, Wolzt M, et al. Effects of insulin on retinal and pulsatile choroidal blood flow in humans. Arch Opthamol 2000;118:55-59.
Polonsky KS. Comment regarding: Intermittent Insulin Therapy. Adolphus Busch Professor and Chairman, Department of Medicine, Director, Institute of Clinical and Translational Sciences, Washington University School of Medicine. August 4, 2009.
Polonsky KS. Lilly Lecture 1994. The beta-cell in diabetes: From molecular genetics to clinical research. Diabetes 1995 Jun;44(6);705-717.
Polonsky KS, Given BD, Hirsch LJ, Tillil H, Shapiro ET, Beebe C, et al. Abnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitus. NEJM 1988 May 12;318(19):1231-1239.
Polonsky KS, Given BD, Prugh W, Licinio-Paixao J, Thompson JE, Karrison T, et al. Calculation of the systemic delivery rate of insulin in normal man. J Clin Endocrinol Metab 1986;63(1):113-118.
Polonsky KS, Sturis J, and Van Cauter E. Temporal profiles and clinical significance of pulsatile insulin secretion. Horm Res 1998;49(3-4):178-184.
Pomposelli JJ, Baxter JK III, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr 1998;22:77-81.
Pørksen N. The in vivo regulation of pulsatile insulin secretion. Diabetologia 2002 Jan;45(1):3-20.
Porksen N, Grofte T, Nyholm B, Holst JJ, Pincus SM, Veldhuis JD, et al. Glucagon-like peptide 1 increases mass but not frequency or orderliness of pulsatile insulin secretion. Diabetes 1998;47:45-49.
Pørksen N, Juhl C, Hollingdal M, Pincus SM, Sturis J, Veldhuis JD, Schmitz O. Concordant induction of rapid in vivo pulsatile insulin secretion by recurrent punctuated glucose infusions. Am. J. Physiol. (Endocrinol Metab) 2000;278:E162-E170.
Pørksen N, Munn S, Steers J, Vore S, Veldhuis J, Butler P. Pulsatile insulin secretion accounts for 70% of total insulin secretion during fasting. Am J Physiol. 269 (Endocrinol Metab) 1995 Mar 27;32:E478-488.
Pørksen N, Nyholm B, Veldhuis JD, Butler PC, Schmitz O. In humans at least 75% of insulin secretion arises from punctuated insulin secretory bursts. Am J Physiol Endocrinol Metab 1997 Jul 22;273:E908-E914.
Porte D Jr. Banting lecture 1990. Beta-cells in type II diabetes mellitus. Diabetes 1991 Feb;40(2):166-180.
Postic C, Shiota M, Magnuson MA. Cell-specific roles of glucokinase in glucose homeostasis. Recent Prog Horm Res 2001;56:195-217. Online at: http://www.rphr.endojournals.org
Pratley RE and Weyer C. Review. The role of impaired early insulin secretion in the pathogenesis of type II diabetes mellitus. Diabetologia 2001 Aug;44(8):929-945.
Prodo Labrotories, Inc. Irvine, California, United States. (Hardcopy available.) Online at: http://www.prodolabs.com/index.html.
Proposed decision by California Administrative Law Judge Steven A. Smith ordering CalPers to pay for Activation. This decision was voted on and approved by the CalPers Board. (Submitted April 20, 2009 with public comments via mail from Michael Bradley, Clinic Manager, Aoki Diabetes Research Institute.)
Public Law 105-33,105th Congress. H.R.2015 Balanced Budget Act of 1997. Online at: http://frwebgate.access.gpo.gov/cgibin/getdoc.cgi?dbname=105_cong_public_laws&docid=f:publ33.105
Pulse Medix. Online at: http://www.pulsemedics.com/
Radetti G, Ghizzoni L, Paganini C, Iughetti L, Caselli G, Bernasconi S. Insulin pulsatility in obese and normal prepubertal children. Horm Res 1998;50(2):78-82.
Ratzmann KF, Schultz B, Heinke P, Michaelis D. Quantitative and qualitative changes in early insulin response to glucose in subjects with impaired carbohydrate tolerance. Diabetes Care 1981;4:85-91.
Rave K, Heise T, Weyer C, Sawicki P, Heinemann L. Measurement of insulin sensitivity: Influence of potassium supply during euglycaemic glucose clamps in healthy volunteers. Exp Clin Endocrinol Diabetes 1999;107:313-317.
Regence Blue Cross and Blue Shield of Utah. Article for PIVIT, Metabolic Activation, or Hepatic Activation Therapy for Diabetes Mellitus (A27223). April 2, 2005. Online at: http://www.cms.gov/mcd/viewarticle_pdf.asp?article_id=27223&article_version=2&contractor_id=39
Ritz R. Resource management for noninvasive monitoring. Resp Care 1990 July;35:728-736.
Ritzel R, Schulte M, Porksen N, Nauck MS, Holst JJ, Juhl C, et al. Glucagon-like peptide 1 increases secretory burst mass of pulsatile insulin secretion in patients with type 2 diabetes and impaired glucose tolerance. Diabetes 2001;50:776-784.
Root HF, Carpenter TM. The effects of the dietary supply of carbohydrate upon the response of the human respiratory quotient after glucose administration. J of Nutrition 1944;333-341.
Rossini AA, Self J, Aoki TT, Goldman RF, Newmark SR, Meguid MM, et al. Metabolic and endocrine studies in a case of lipoatrophic diabetes. Metabolism 1977 Jun;26(6):637-650.
Saudek CD. Novel forms of insulin delivery. Endocrinology and Metabolism Clinics of North America 1997 September;26(3):599-610.
Schmitz O, Arnfred J, Hielsen OH, Beck-Nielsen H, Orskov H. Glucose uptake and pulsatile insulin infusion: euglycaemic clamp and [3-3H] glucose studies in healthy subjects 1986 Dec;113(4):559-563.
Schmitz O, Pedersen SB, Mengel A, Pørksen N, Bak J, Møller N, et al. Augmented effect of short-term pulsatile versus continuous insulin delivery on lipid metabolism but similar effect on whole-body glucose metabolism in obese subjects. Metab 1994 Jul;43(7):842-846.
Scott D. Fisher A. The effect of zinc salts on the action of insulin. J. Pharmacol. Exp. Ther 1935;55: 206.
Scott JF, Robinson GM, French JM, O’Connell JE, Alberti KG, Gray CS. Glucose potassium insulin infusions in the treatment of acute stroke patients with mild to moderate hyperglycemia: the glucose insulin in stroke trial (GIST). Stroke 1999;30:793-799.
Scottish Intercollegiate Guideline Network (SIGN).
(The SIGN does not have any guidelines that address use of this therapeutic modality or the use of calorimetry in diabetic patients.)
(The SIGN has a single guideline regarding diabetes. Multiple other earlier guidelines on various aspects of diabetes were superseded by guideline #55.)
Management of Diabetes #55 (Issued 2001; reviewed 2005; need for update delineated)
Securities Exchange Commission (SEC). Amendment to Registration of Securities. Online at: http://www.secinfo.com/d11Eeb.4f896.htm; and http://www.secinfo.com/d11Eeb.4f89d.b.htm (See ADRI. See Diabetex.)
Seoane J, Gómez-Foix AM, O’Doherty RM, Gómez-Ara C, Newgard CB, Guinovart JJ. Glucose 6-Phosphate produced by glucokinase, but not hexokinase I, promotes the activation of hepatic glycogen synthase. J Biol Chem 1996 September 27;271(39):23756-23760.
Shah P, Vella A, Basu A, Basu R, Schaewk WF, Rizza RA. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin endocrinol Metab 2000 Nov;85(11):4053-4059.
Shapiro ET, Tillil H, Rubenstein AH, Polonsky KS. Peripheral insulin parallels changes in insulin secretion more closely than C-peptide after bolus intravenous glucose administration. J Clin Endocrinolol Metab 1988;67(5):1094-1099.
Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, et al. Care of children and adolescents with type 1 diabetes: A statement of the American Diabetes Association. Diabetes Care. 2005. 28:186-212. Online at: http://care.diabetesjournals.org/content/28/1/186.full
Simmons D, Morton K, Laughton SJ, Scott DJ. A comparison of two intravenous insulin regimens among surgical patients with insulin-dependent diabetes mellitus. Diabetes Educ 1994 Sep-Oct;20(5):422-427.
Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale E, et al. Intensive glycemic control and the prevention of cardiovascular events: Implications of the ACCORD, ADVANCE, and VA diabetes trials. A position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation 2009 Jan 20;119:351-357.
Smith CP, Tarn AC, Thomas JM, Overkamp D, Corakci A, Savage MO, et al. Between and within subject variation of the first phase insulin response to intravenous glucose. Diabetologia 1988 Feb;31(2):123-125.
Social Security Act, Section (§)1861(s)(2)(C) (diagnostic tests – other), a Part B benefit. Online at: http://www.ssa.gov/OP_Home/ssact/title18/1861.htm
Song, SH, McIntyre SS, Shah H, Veldhuis JD, Hayes PC, Butler PC. Direct measurement of pulsatile insulin secretion from the portal vein in human subjects. J Clin Endocrinol Metab. 2009 February 24;85(12):4491-4499.
Song H, Huewang L, Chuanyou Z, Min W. Glomerulosclerosis in Adriamycin-induced nephrosis is accelerated by a lipid-rich diet. Pediatr Nephrol 2000 July 14;15:196-200.
Stagner JI, Samols E, Weir GC. Sustained oscillations of insulin, glucagon, and somatostatin from the isolated canine pancreas during exposure to a constant glucose concentration. J Clin Invest 1980 Apr;65:939-942.
Strategic Partners – Bionica. (See VitalCare) (See Diabetes.net)Online at: http://www.vitalcaretechnology.com/Pdf/Strategic Partners feb2908.pdf
Sturis J, Polonsky KS, Shapiro ET, Blackman JD, O’Meara NM, Van Cauter E. Abnormalities in the ultradian oscillations of insulin secretion and glucose levels in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1992;35:681-689.
Takeuchi H, Inoue Y, Ishihara H, Oka Y. Overexpression of either liver type of pancreatic beta cell type glucokinase via recombinant adenovirus enhances glucose oxidation in isolated rat hepatocytes. FEBS Lett. 1996 September 9;393(1):60-64.
Talbot G.. Histology of Bowman’s membrane in cases of glaucoma. Br J Opthalmol 1938 Apr;22(4):210-214.
Tannenbaum GS, Martin JB, Colle E. Ultradian growth hormone rhythm in the rat: effects of feeding, hyperglycemia, and insulin-induced hypoglycemia. Endocrinol 1976 Sept;99(3):720-727.
Tattersall RB. A paper which changed clinical practice (slowly). Jacob Holler on potassium deficiency in diabetic acidosis (1946). Diabet Med 1999 Dec;16(12):978-984.
The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. NEJM 2008 Jun 12;358(2):2545-2559.
The Management of Diabetes Mellitus Working Group. VHA/DoD clinical practice guideline for the management of diabetes mellitus in the primary care setting. Contract number: V101(93)P-1633. 1999 December:(2.2).
Thiebaud D, Schutz Y, Acheson K, Jacot E, DeFronzo RA, Felber J, et al. energy cost of glucose storage in human subjects during glucose-insulin infusions. American Physiological Society 1983:E216-E221.
Thorens B. GLUT2 in pancreatic and extra-pancreatic gluco-detection (review). Mol Membr Biol 2001 Oct-Dec;18(4):265-273.
Tilley JW. Glucokinase activators: Novo Nordisk’s WO2008084044 and Array Biopharma’s WO2008091770. Expert Opin Ther Pat 2009;19(4): 549-553.
Torres TP, Catlin RL, Chan R, Fujimoto Y, Sasaki N, Printz RL, et al. Restoration of hepatic glucokinase expression corrects hepatic glucose flux and normalizes plasma glucose in Zuker diabetic fatty rats. Diabetes 2009 January;58:78-86.
Torres N, Noriega L, Tovar AR. Nutrient Modulation of insulin secretion. Chapter nine. Vitamins and Hormones. Elsevier Inc., Amsterdam, The Netherlands. 2009;80:217-244.
Trivedi HS, Pang MM, Campbell A, Saab P. Slowing the progression of chronic renal failure: economic benefits and patients’ perspectives. Am J Kidney Dis 2002 Apr;39(4):721-729.
Truong MP, Arcangeli MA, Benbarka MM, Aoki TT, Grecu EO. The impact of Chronic Intermittent Intravenous Insulin Therapy (CIIIT) on blood pressure inpatients with IDDM and hypertension. Clin Res 1993;41:65A.
Tzamaloukas AH, Oreopoulos DG. Subcutaneous versus intraperitoneal insulin in the management of diabetics on CAPD: A review. Adv Perit Dial 1991;7:81-85.
Turnheim K, Waldhäusl WK. Essentials of insulin pharmacokinetics. Wien Klin Wochenshr 1988 Feb 5;100(3):65-72.
Uchino H, Niwa M, Shimizu T, Nishiyama K and Kawamori R. Impairment of early insulin response after glucose load, rather than insulin resistance, is responsible for postprandial hyperglycemia seen in obese type 2 diabetes: assessment using nateglinide, a new insulin secretagogue. Endocrine J 2000;47(5):639-641.
United Healthcare Diabetic 017.1 T3. Intermittent Intravenous Insulin Therapy. Noncoverage. Online at: https://www.oxhp.com/secure/policy/intermittent_iv_insulin_therapy_309.html
UK Prospective Diabetes Study (UKPDS) Group. [No authors listed]. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications inpatients with type 2 diabetes (UKPDS 33). Lancet 1998 Sept 12;352:837-853.
United Kingdom Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998 Sept 12;352:854-865.
United States (US) Patent 6582716 – Method for treating wounds, promoting healing and avoiding amputations in diabetic and non-diabetic patients. Inventor: Aoki, Thomas T. Online at: http://www.patentgenius.com/patent/6582716.html
United States (US) Patent 6967191. System for treating eye and nerve diseases in diabetic and non-diabetic patients. Inventor: Aoki, Thomas T. Publication Date: 11/22/2005. Filing Date 3/19/2003. Online at: http://www.freepatentsonline.com/6967191.html
Utilization (See CCI letter.) (See ATS/ACCP letter.)
Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels: Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41(3):368-377.
Van den Berghe G, Wouters PJ, Bouillon R, et al. Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit Care Med 2003 Feb;31(2):359-366.
Van Loan MD. Do hand-held calorimeters provide reliable and accurate estimates of resting metabolic rate? J Am College of Nutrition.2007;26(6):625-629.
Verdin E, Castillo M, Luyckx AS, Lefebvre P. Similar metabolic effects of pulsatile versus continuous human insulin delivery during euglycemic, hyperinsulinemic glucose clamp in normal man. Diabetes 1984 Dec;33(12):1169-1174.
Vessby B, Karlström B, Ohrvall M, Järvi A, Andersson A, Basu S. Diet, nutrition and diabetes mellitus. Ups J Med Sci 2000;105(2):151-160.
Vester JW, Reino ML. Hepatic Glucokinase: A direct effect of insulin. American Association for the Advancement of Science1963 November 1;142(3592):590-591.
Veterans Health Administration (VHA)
(The VHA does not have any guidelines that address use of the outpatient intravenous insulin therapeutic modality or the use of calorimetry in diabetic patients.)
Veterans Health Administration (VHA) Department of Defense (DoD)
Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting: Veterans Health Administration Department of Defense. 1999. Online at: http://www.google.com/search?q=VHA/DOD+Clinical+Practice+Guideline+for+the+management+of+Diabetes+Mellitus+in+the+Primary+Care+Setting
Veterans Health Systems. Pulsatile insulin therapeutic program. (Personal communication with Dr. Leonard Pogach, Director, Veterans Administration, New Jersey Healthcare System, Center for Healthcare Knowledge Management, March 2009.)
Vialetes B, Mattei-Zevaco C, Badier C, Ramahandridona G, Lassmann-Vague V, Vague PH. Low acute insulin response to intravenous glucose. A sensitive but non-specific marker of early stages of type 1 (insulin-dependent) diabetes. Diabetologia 1988 Aug;31(8):592-596.
Viberti G, Mogensen VG, Groop LC, Pauls JF. Effect of captopril on the progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria JAMA 1994; 271-275-279.
Vinik. Office of Human Research Protection (OHRP) Sanction. Online at: http://www.hhs.gov/ohrp/detrm_letrs/YR06/jun06a.pdf
VitalCare. (See Inside Wall Street Report) (See Diabetes.net) (See Strategic Partners) Online at: http://vitalcaretechnology.com/strategic-partners.htm.
Vlachokosta FV, Asmal AC, Ganda OP, Aoki TT. The effect of strict control with the artificial beta-cell on plasma lipid levels in insulin-dependent diabetes. Diabetes Care 1983 Jul-Aug;6(4):351-355.
Ward GM, Marangou AG, Best JD, Aitken PM, Alford FP. Effects of short-term pulsatile and continuous insulin delivery on glucagon secretion and insulin secretion and action. Metab 1989 Apr;38(4):297-302.
Ward GM, Walters JM, Aitken PM, Best JD, Alford FP. Effects of prolonged pulsatile hyperinsulinemia in humans: Enhancement of insulin sensitivity. Diabetes 1990;39:501-507.
Watanabe RM, Volund A. Roy S. Bergman RN. Prehapeatic beta-cell secretion during the intravenous glucose tolerance test in humans: application of a combined model of insulin and C-peptide kinetics. J Clin Endocrinol Metab 1989 Oct;69(4):790-797.
Wathion N. Public statement on Exubera (Insulin Human) withdrawal of the marketing authorization in the European union. Drugs.com. London. November 10, 2008. Online at: http://www.drugs.com/news/public-statement-exubera-insulin-human-marketing-authorisation-european-union. 14661.html
Watkins K, Connell, CM. Measurement of health-related QOL in diabetes mellitus. PharmacoEconomics 2004;22(17):1109-1126.
Watkins P. Editor. The UKPDS: A model for gathering the evidence for the management of chronic diseases. J of the Royal College of Physicians of London 1998 November/December;32(6):510-511.
Webster GK, Bell RG. Gas chromatographic analysis of fosfomycin in plasma for pharmacokinetic analysis. J AOAC Int 1999 May-Jun;82(3):620-624.
Webster PA, King SE, Torres A. An in vitro validation of a commercially available metabolic cart using pediatric ventilator volumes. Pediatric Pulmonology 1998;26:405-411.
Wiederkehra, Wollheim CB. Impact of mitochrondrial calcium on the coupling of metabolism to insulin secretion in the pancreatic ß-cell. Cell Calcium.2008;44:64-76.
Weigle DS, Rumbaoa AV, Goodner CJ. Lack of evidence for improvement in long-term glycemic control by pulsatile insulin infusion in streptozocin-induced diabetic baboon. Diabetes 1991 Mar;40(3):349-357.
Weigle DS. Pulsatile secretion of fuel-regulatory hormones. Diabetes 1987 Jun;36(6):764-775.
Weinrauch LA, Bayliss G, Gleason RE, Lee aT, D’Elia JA. A pilot study to assess utility of changes in elements of the diabetes impact management scale in evaluating diabetic patients for progressive nephropathy. Metab Clin Exp 2009A;58:492-496.
Weinrauch LA, Bayliss G, Gleason RE, Lee AT, D’Elia JA. Utilization of an abbreviated diabetes impact management scale to assess change in subjective disability during a trial of pulsatile insulin delivery demonstrates benefit. Metab Clin Exp 2009B;58:488-491.
Weinrauch LA, Burger AJ, Aepfelbacher F, Lee AT, Gleason RE, Elia JA. A pilot study to test the effect of pulsatile insulin infusion on cardiovascular mechanisms that might contribute to attenuation of renal compromise in type 1 diabetes mellitus patients with proteinuria. Metab Clin Experimental 2007;56(11):1453-1457.
Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. Nutrition 1990 May-Jun; 6(3):213-221.
Wells C. Integrated Care. Emergency Ward Zen. Health Service Journal 1998 Nov 26;108(5632):32-33.
Wells JCK. Is obesity really due to high energy intake of low energy expenditure? International Journal of Obesity 1998;22:1139-1140.
Wesson DE, Black PR, Vlachokosta F, Aoki TT, Wilmore DW. Artificial beta-cell promotes positive nitrogen balance and whole body protein synthesis in insulin-dependent diabetic subjects. JPEN J Parenter Enteral Nutr 1988 May-Jun;12(3):237-243.
Wilmshurst EG, Soeldner JS, Holsclaw DS, Kaufmann RL, Shwachman H, Aoki TT, et al. Endogenous and exogenous insulin responses in patients with cystic fibrosis. Pediatrics 1975 Jan;55(1):75-82.
Wolfsdorf JI, MB, BCh, Editor. Intensive diabetes management. 4th Edition. American Diabetes Association 2009.
Woolfson AM. Techniques, Materials, and Devices: An improved method for blood glucose control during nutritional support JPEN J Parenter Enteral Nutr 1981;5(5):436-440.
Woolfson AM. Control of blood glucose during nutritional support in ill patients. Intensive Care Med 1980;7:11-14.
Yadav N, Morris G, Harding WE, Ang S, Adams GG. Various non-injectable delivery systems for the treatment of diabetes mellitus. Endocr Metab Immune Disord Drug Targets 2009 Mar;9(1):1-13.
Žarkovic M, Ciric J, Penezic Z, Trbojevic B, Drezgic M. Effect of weight loss on the pulsatile insulin secretion. J of Clin Endocrinol Metab.2000 July 12;85(10):3673-3677.
Žarkovic M, Ciric J, Stojanovic M, Penezic Z, Trbojevic B, Drezgic M, et al. Effect of insulin sensitivity on pulsatile insulin secretion. European J of Endocrinol 1999;141:495-501.
Zerr KJ, Furnary aP, Grunkemeier GL, Bookin S, Kanhere V, Starr A. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg 1997;63:356-361.
Zhang Y. Predicting occurrences of acute hypoglycemia during insulin therapy in the intensive care unit. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society, Conference. Vancouver, British Columbia, Canada. 2008 August 20;24:3297-3300.
Appendix C
Citations and Submissions by CommentersAmerican Association of Clinical Endocrinologists Report, 2007.
American Diabetes Association. Clinical Practice Recommendations 2003. Diabetes Care. 2003;26(Suppl 1).
American Diabetes Association Clinical Practice Recommendations.
Am J Hypertension. 1995;8:782-789.
Am J Hypertension 1997;10:454-461.
Am J Cardiology. 1997;80: 1198-1202.
Am J Hypertension. 1998;11(3): 302-308.
Am J Hypertension 1999;12:1135-1139.
American Journal of Cardiology 1999;84:449-453.
American Journal of Cardiology 1999;84:687-691.
Aoki’s, M.D. Response to Blue Shield.
Aoki TT, Benbarka MM. Type I diabetes: An intensive approach to controlling blood glucose levels. Modern Medicine. 1992;60:88-103.
Aoki, TT, Benbarka MM. Type I diabetes: The ‘sleeping liver’ hypothesis and its clinical implications. Modern Medicine. 1992;60:73-76.
Aoki TT, Benbarka MM, Oklmura MC, Arcangeli MA, Walter RM, Wilson LD, et al. Long-term intermittent intravenous insulin therapy and type 1 diabetes mellitus. Lancet. 1993;342(8870):515-518.
Aoki TT, Grecu EO, Arcangeli MA. Chronic intermittent intravenous therapy corrects orthostatic hypotension of diabetes. Am J of Medicine. 1995;99(6):683-684.
Aoki TT, Grecu EO, Arcangeli MA. Effect of intensive intravenous insulin therapy on IGF-1 and IGFBP-1 blood levels inpatients with IDDM. J Investigative Med. 1997;45(1):135A.
Aoki TT, Grecu EO, Arcangeli MA. Reversal of severe nonschemic dilated cardiomyopathy by intensive intravenous insulin therapy in a patient with NIDDM. J Investigative Med. 1996;44(1): 126A.
Aoki TT, Grecu EO, Arcangeli MA, Benbarka MM, Prescott P, Ahn JH. Chronic intermittent intravenous insulin therapy: a new frontier in diabetes therapy. Diabetes Technol Ther. 2001. Spring; 3(1):111-123. Review.
Aoki TT, Grecu EO, Gollapudi GM, Barber, AR, Arcangeli MA, Benbarka MM, et. al. Effect of intensive insulin therapy on progression of overt nephropathy inpatients with type 1 diabetes mellitus. Endocrine Practice. 1999;5(4):174-178.
Aoki TT, Grecu EO, Meisenheimer. Effect of intensive insulin therapy of abnormal circadian blood pressure pattern inpatients with type 1 diabetes mellitus. Online J Curr Clin Trials; 1995. Dec 15; Doc No 199.
Aoki TT, Grecu EO, Prendergast JJ, Arcangeli MA, Meisenheimer R. Effect of chronic intermittent intravenous insulin therapy on antihypertensive medication requirement in IDDM subjects with hypertension and nephropathy. Diabetes Care. 1995;18(9):1260-1265.
Aoki TT, Viachokosta FV, Foss MC, Meistas MT. Evidence for restoration of hepatic glucose processing in type 1 diabetes mellitus. J Clin Invest. 1983;71(4):837-839.
Arch Int Med 2001;161:98-101.
Bando. Results of Clinical Trial of Five Patients Treated for One and a half Years With MAT™ Treatment. May 25, 2007. Fukui, Japan. English Translation Powerpoint.(on-line submission can be found at: F:\My Projects\Diabetes\Metabolic Activated Therapy CAG 00410N\NCA\Dr Bando's Presentation JDA 25 May 2007 English.PPT)
Bass FM. "The adoption of a marketing model: Comments and observations". In V. Mahajan & Y. Wind (Eds.), Innovation Diffusion Models of New Product Acceptance. Cambridge, Mass.: Ballinger, 1986.
Blue Shield’s review of hepatic activation.
California Court of Appeals upholding the Superior Court decision.
Ca Public Employees’ Retirement System (CalPERS): Board of Administration. In the matter of the consolidated appeals of denial of coverage for hepatic activation treatment of: Names withheld (5). Case No.: 3490-5, 3490-3, 3490-2, 3490-1, 3490-4 and 3490-6.
CalPERS Court Ruling 2002. (on-line version submitted and can be found at: F:\My Projects\Diabetes\Metabolic Activated Therapy CAG 00410N\NCA\Calpers Decision\CalPers Decision 4 30 02.pdf)
CalPERS Proposed Decision 1.17.02 (on-line version submitted and can be found at: F:\My Projects\Diabetes\Metabolic Activated Therapy CAG 00410N\NCA\Calpers Decision\CalPers Proposed Decision 1 17 02.pdf)
CalPERS Judgement (on-line version submitted and can be found at: F:\My Projects\Diabetes\Metabolic Activated Therapy CAG 00410N\NCA\Calpers Decision\judgment.pdf)
CalPERS Order Re Legal Issues (on-line version submitted and can be found at: F:\My Projects\Diabetes\Metabolic Activated Therapy CAG 00410N\NCA\Calpers Decision\Order Re Legal Issues.pdf)
CalPERS Legal Issue 6 (on-line version submitted and can be found at: F:\My Projects\Diabetes\Metabolic Activated Therapy CAG 00410N\NCA\Calpers Decision\Order Legal Issue 6.pdf)
Christenson, C. The Innovator’s Dilemma: When new technologies cause great firms to fail. Harvard Business School Press 1997.
Dailey GE, Boden GH, Creech RH, Johnson DG, Gleason RE, Kennedy FP, et al. Effects of pulsatile intravenous insulin therapy (PIVIT) on the progression of diabetic nephropathy. Metabolism. 2000;49(11):1491-1495.
DCCT(USA).
Field N, Boe N, Gilbert W, Benbarka M, Aoki T. The effect of chronic intermittent intravenous insulin therapy on pregnancy outcome in insulin dependent diabetes mellitus. J Soc Gynecol Invest. 1997;4(1, suppl): 196A.
Foss, MC, Vlachokosta FV, Cunningham LN, Aoki TT. Restoration of glucose homeostasis in insulin-dependent diabetic subjects. An inducible process. Diabetes. 1982;31(1):46-52.
Hayes Report, 2006.
Healthy People 2000 Review (1998-99). http://www.health.gov/healthypeople
Heinemann L. Comment regarding: Pulsatile Insulin Therapy. Profil Institute for Metabolic Resarch GmbH. Neuss, Germany. June 15, 2009.
Heinemann L, Sonnenberg GE, Hohmann A, Ritzenhoff A, Berger M, Benn J, et al. Pulsatile insulin infusion and glucose-homeostasis in well-controlled type 1 (insulin-dependent) diabetic patients. J Intern Med. 1989. Nov;226(5);325-333.
International Journal of Cardiology. 2002;86:281-287.
International Journal of Cardiology. 2004;94: 47-51.
Journal of Clinical Hypertension. 2005;7:159-164.
Journal of Clinical Hypertension. 2006;8:330-335.
Lew’s assessment of the statistical design of three studies on hepatic activation.
Logan-Darrough M. Pulsatile I.v. insulin therapy for severely out of control diabetes. J Intraven Nurs. 1995;May-Jun;18(3):124-128.
MAT Protocol.
Meistas MT, Vlachokosta FV, Gleason RE, Arcangeli M, Aoki TT. Role of muscle in C02 production after oral glucose administration in man. Diabetes. 1985;34(10):960-963.
Metabolism. 2000. 49:88-91.
Metabolism. 2009
Milstein B, Jones A, Homer J. Charting Plausible Futures for Diabetes Prevalence in the United States: A Role for System Dynamics Simulation Modeling. Prev. Chronic Dis. 2007. July; 4(3):A52.
Mirbolooki M, Taylor GE, Knutzen VK, Scharp DW, Willcourt R, Lakey JRT. Pulsatile Intravenous Insulin Therapy: The Best Practice to Reverse Diabetes Complications? Medical Hypotheses. 2009 (In Press).
Moore G. Crossing the chasm: Marketing and selling high-tech products to mainstream customers (1991, revised 1999, 2000, 2001, 2002) New York: Harper Collins. Ohkubo(Japan).
Proposed decision by California Administrative Law Judge Steven A. Smith ordering CalPers to pay for Activation. This decision was voted on and approved by the CalPers Board.
Rogers EM. Diffusion of innovations (2003, 5th ed.). New York: Free Press.
Superior Court of California decision, ordering Blue Shield to pay for MAT therapy.
Trivedi HS, Pang MM, Cambell A, Saab P. Slowing the progression of chronic renal failure: economic benefits and patients’ perspectives. Am J Kidney Dis. 2002. 39(4):721-729.
UKPDS(UK)
Weinrauch LA, Bayliss G, Gleason RE, Lee AT, D"Elia JA. Utilization of an abbreviated diabetes impact management scale to assess change in subjective disability during a trial of pulsatile insulin delivery demonstrates benefit. Metabolism. 2009. 58(4):488-491.
Weinrauch LA, Bayliss G, Gleason RE, Lee AT, D"Elia JA. A pilot study to assess utility of changes in elements of the Diabetes Impact Management Scale in evaluating diabetic patients for progressive nephropathy. Metabolism. 2009. Apr;58(4):492-496.
Weinrauch LA, Burger A, Aepfelbacher F, Lee A, Gleason RE, D"Elia JA. A pilot study to test the effect of pulsatile insulin infusion on cardiovascular mechanisms that might contribute to attenuation of renal compromise in type 1 diabetes mellitus patients with proteinuria. Metabolism: Clinical and Experimental. 2007. 56(11):1453-1457.
WellPoint Medical Policy and Technology Assessment Committee (MPTAC) review of Chronic Intermittent Intravenous Insulin Therapy (CIIT), July 2008
Appendix D:
Clinical Trials Listed on ClinicalTrials.gov Website
(http://clinicaltrials.gov/ct2/results?term=pulsatile+insulin; Accessed 4/19/09)
| Study/PI |
Subject |
Diabetes |
Blinding |
Randomized |
Control |
Duration |
Enrollment |
NCT00228891 Tuller |
Neuropathy Endpoint(s) not described |
Type 1 & 2 W refractory neuropathy Can be on oral rx |
Investigator |
No |
Assigned |
Not stated Q 6 mo tests 12 mo w 6 mo renewals |
Started 2005 200 initially 500 |
NCT00228904 Tuller |
Neuropathy via survey & NCV & quantitative sensory testing & cardiac beat-to-beat variability |
Type 1 & 2 W refractory neuropathy |
Investigator |
No |
Assigned |
Not stated Q 6 mo tests |
Started 2005 200 initially 400 |
NCT00287651 Tuller |
Retinopathy via photos & Hopkins evaluation |
Type 1 & 2 W PDR, NPDR, ME eye dx |
Investigator |
No |
Assigned |
Not stated Q 3-6 mo tests |
Started 2005 300 |
NCT00539435 Tuller |
Cardiac Cardiac QOL vs carotid US, ECHO, labs |
Unspecified w cardiac dx+ other complications Can be on oral rx |
Open |
No |
Unclear |
Not stated Q 6 mo tests 12 mo & 6 mo renewals |
Started 2007 400 |
NCT00361907 Tuller |
Risk Markers (Multiple) |
Type 1 & 2 W complications |
Investigator |
No |
Assigned |
6 mo extended to =2 yrs |
Started 2005 200 initially 500 |
NCT00228878 Tuller |
QOL (DMIS) Not in protocol |
Type 1 & 2 Can be on oral rx |
Open |
No |
Pre-tx |
Not stated |
Started 2003 200 initially 500 |
NCT00539409 Tuller |
Metabolic Integrity via QOL & lab tests Not in protocol |
Unspecified w complications Can be on oral rx |
Open |
No |
Pre-tx |
Not stated Q 6 mo tests |
Started 2006 750 |
NCT00228865 Tuller |
Cognitive Function |
Type 1 & 2 |
Open |
No |
Pre-tx |
6-12 mo w 8 6 mo renewals* |
2003-6 Complete Not published 50 |
NCT00594152 Dailey |
Nephropathy via renal, cardiac, neurologic, retinal tests |
Type 1 W proteinuria |
Open |
Yes |
Yes |
12 mo Extended to 18 mo |
1993-5 Listed 1/08 Complete Published 2000 71 |
DMIS=Diabetes measurement Impact scale. See 2009 Weinrauch studies.
DQOL= Diabetes Quality of Life survey
Dx=disease
ME=macular edema
Mo=month
NCV=nerve conduction velocity studies
NPDR non-proliferative retinopathy
PDR=proliferative diabetic retinopathy |
PI=principal investigator
Q=each
QOL=Quality of life
Rx=medication
W=with
8=infinite or indefinite
*found only in archived protocol |


