To: Administrative File: CAG-00063R3
From: Louis Jacques, MD
Director, Coverage and Analysis Group
Tamara Syrek Jensen, JD
Deputy Director, Coverage and Analysis Group
Jyme Schafer, MD, MPH
Director, Division of Medical and Surgical Services
Joseph Chin, MD, MS
Lead Medical Officer
Jamie Hermansen, MPP
Lead Health Policy Analyst
Subject: Decision Memorandum for Reconsideration of Coverage of Cardiac Pacemakers:
Single Chamber and Dual Chamber Permanent Cardiac Pacemakers.
Date: August 13, 2013
I. Decision
The Centers for Medicare & Medicaid Services (CMS) has determined that the evidence is sufficient to conclude that implanted permanent cardiac pacemakers, single chamber or dual chamber, are reasonable and necessary for the treatment of non-reversible symptomatic bradycardia due to sinus node dysfunction and second and/or third degree atrioventricular block. Symptoms of bradycardia are symptoms that can be directly attributable to a heart rate less than 60 beats per minute (for example: syncope, seizures, congestive heart failure, dizziness, or confusion).
Therefore, the following indications are covered for implanted permanent single chamber or dual chamber cardiac pacemakers:
- Documented non-reversible symptomatic bradycardia due to sinus node dysfunction.
- Documented non-reversible symptomatic bradycardia due to second degree and/or third degree atrioventricular block.
The following indications are non-covered since there is insufficient evidence to conclude that implanted permanent cardiac pacemakers, single chamber or dual chamber, are reasonable and necessary:
- Reversible causes of bradycardia such as electrolyte abnormalities, medications or drugs, and hypothermia.
- Asymptomatic first degree atrioventricular block.
- Asymptomatic sinus bradycardia.
- Asymptomatic sino-atrial block or asymptomatic sinus arrest.
- Ineffective atrial contractions (e.g., chronic atrial fibrillation or flutter, or giant left atrium) without symptomatic bradycardia.
- Asymptomatic second degree atrioventricular block of Mobitz Type I unless the QRS complexes are prolonged or electrophysiological studies have demonstrated that the block is at or beyond the level of the His Bundle (a component of the electrical conduction system of the heart).
- Syncope of undetermined cause.
- Bradycardia during sleep.
- Right bundle branch block with left axis deviation (and other forms of fascicular or bundle branch block) without syncope or other symptoms of intermittent atrioventricular block.
- Asymptomatic bradycardia in post-myocardial infarction patients about to initiate long-term beta-blocker drug therapy.
- Frequent or persistent supraventricular tachycardias, except where the pacemaker is specifically for the control of the tachycardia.
- A clinical condition in which pacing takes place only intermittently and briefly, and which is not associated with a reasonable likelihood that pacing needs will become prolonged.
Medicare Administrative Contractors will determine coverage under section 1862(a)(1)(A) of the Social Security Act for any other indications for the implantation and use of single chamber or dual chamber cardiac pacemakers that are not specifically addressed in this national coverage determination.
II. Background
Throughout this document we use numerous acronyms, some of which are not defined as they are presented in direct quotations. Please find below a list of these acronyms and corresponding full terminology.
ACC – American College of Cardiology
ACCF – American College of Cardiology Foundation
AF – atrial fibrillation
AHA – American Heart Association
AV – atrioventricular
AVB – atrioventricular block
BPEG – British Pacing and Electrophysiology Group
bpm – beats per minute
CTOPP – Canadian Trial of Physiologic Pacing
HAS – Haute Autorité de Santé (French: High Authority for Health)
HRS – Heart Rhythm Society
LA – left atrial
LV – left ventricular
LVFS – left ventricular fractional shortening
MOST – Mode Selection Trial
NASPE – North American Society of Pacing and Electrophysiology (now known as the Heart Rhythm Society)
PASE – Pacemaker Selection in the Elderly
PR – pulse rate
RCT – randomized controlled trial
SND – sinus node dysfunction
SSS – sick sinus syndrome
UKPACE – United Kingdom Pacing and Cardiovascular Events
Nomenclature for cardiac pacemaker mode selection is also referenced throughout this document. The nomenclature was developed by the North American Society of Pacing and Electrophysiology (now known as the Heart Rhythm Society (HRS)) and the British Pacing and Electrophysiology Group (NASPE/BPEG). The pacing mode codes currently in use were originally published in 1987 and updated in 2002. Positions I, II, and III indicate the heart chambers where pacing and sensing occur. Position IV is used to indicate the presence or absence of an adaptive-rate mechanism, and Position V indicates whether multi-site pacing is present (Bernstein et al., 2002) (see Appendix E).
CMS initiated this current national coverage analysis to reconsider coverage indications for single chamber and dual chamber cardiac pacemakers. The scope of this reconsideration and this decision memorandum does not address biventricular pacemakers, pacemakers that stimulate more than two heart chambers, those devices used to treat tachyarrhythmias and cardiac dissynchrony, cardiac resynchronization therapy, cardiac pacemaker evaluation services, or self-contained pacemaker monitors.
Permanent cardiac pacemakers refer to a group of self-contained, battery operated, implanted devices that send electrical stimulation to the heart through one or more implanted leads. Pacemakers are most often used to treat chronic cardiac arrhythmias (abnormalities in the rate and rhythm of heart beats) such as bradycardia (a heart rate usually less than 60 beats per minute (bpm)) or tachycardia (a heart rate faster than 100 or more bpm) that are pathologic. Bradycardia that causes symptoms such as syncope (“a sudden and brief loss of consciousness associated with a loss of postural tone, from which recovery is spontaneous,” Kapoor, 2000) is common in older adults and accounts for a large proportion of permanent pacemaker implantations. Horgan noted in 1984 that “[i]ndeed, symptomatic bradycardia is one of the most important signs of the need for a pacemaker and is considered to be present when symptoms can be directly attributed to a slow heart rate.” Zhan noted in 2008 that patients 65 years or older accounted for over 85 percent of pacemaker implantations.
Bradycardia can be generally divided into sinus node dysfunction (SND) and atrioventricular (AV) conduction disturbances or blocks (AVB) (Mangrum and DiMarco, 2000). In the normal heart beat, the sinus node depolarizes and initiates atrial contraction (P wave on the electrocardiogram). Depolarization travels from the atrium through the AV node into the Bundle of His and the bundle branches, initiating ventricular contraction (QRS complex) (Noble, Hillis and Rothbaum, 1990; see Appendix D). Together SND and AVB account for the majority of implantations of permanent cardiac pacemakers (86 percent (Bernstein, 2001)).
SND, also known as sick sinus syndrome (SSS), may involve abnormalities in the sinus node itself or exit blocks and may be due to intrinsic disease of the nodal tissue due to degeneration or infarction or extrinsic factors such as medications. SND is relatively common in adults 65 years or older (Mangrum and DiMarco, 2000). The American Heart Association (AHA) reported that “[s]inus node dysfunction occurs in 1 of every 600 cardiac patients 65 years of age and accounts for 50% of implantations of pacemakers in the United States (Roger, 2012).” AV conduction disturbances refer to abnormalities (delay or block) in the AV node or conduction bundle or branches and may occur after myocardial infarction or infections. These may occur at “one of two levels: at the level of the AV node or beyond the bifurcation of the bundle of His—that is, at the level of the bundles or ventricle (Noble, Hillis and Rothbaum, 1990).”
AVB is further divided into first degree, second degree and third degree. First degree AVB is an electrocardiographic finding of a prolonged pulse rate (PR) (P wave to R wave) > 0.2 second (see Appendix D) and “does not by itself cause bradycardia, but it is often seen in conjunction with second-degree or third-degree block or sinus-node dysfunction (Mangrum and DiMarco, 2000).” Second degree AVB occurs “when an organized atrial rhythm fails to conduct to the ventricle in a 1:1 ratio but some atrial–ventricular relation is maintained (Mangrum and DiMarco, 2000).” Second degree AVB may be Mobitz type I (also known as Wenckebach block where “the electrocardiogram shows a stable PP interval and a progressive increase in the PR interval until a P wave fails to conduct”) or Mobitz type II (where “there is a stable PP interval with no measurable prolongation of the PR interval before an abrupt conduction failure”) (Mangrum and DiMarco, 2000). Ginter described advanced second degree AVB as follows: “In advanced second-degree AV block, two or more consecutive P waves are blocked” and further explained that “a conduction ratio of 2:1 makes distinguishing between type I or type II difficult. These blocks and those with a conduction ratio of 3:1 or higher are categorized as advanced (Ginter JF, 2011).” Third degree AVB, or complete heart block, refers to instances where “atrial activity and ventricular activity are independent of each other (Mangrum and DiMarco, 2000).”
In the United States, implanted permanent cardiac pacemakers have been used to treat bradycardia since the early 1960s (Chardack, Gage and Greatbatch, 1960). They are often classified by the number of chambers of the heart that the devices stimulate (pulse or depolarize). Single chamber pacemakers typically target either the right atrium or right ventricle. Dual chamber pacemakers stimulate both the right atrium and right ventricle.
The implantation procedure is typically performed under local anesthesia and requires only a brief hospitalization. A catheter is inserted into the chest and the pacemaker's leads are threaded through the catheter to the appropriate chamber(s) of the heart. The surgeon then makes a small "pocket" in the pad of flesh under the skin on the upper portion of the chest wall to hold the power source. The pocket is then closed with stitches. The procedure leaves a small scar and the battery can be felt through the skin.
With technological advancements, the use of dual chamber permanent cardiac pacemakers has dramatically increased over the past several years. Greenspon and colleagues reported that overall use of pacemakers increased by 55.6 percent between 1993 and 2009 with dual chamber pacemakers increasing from 62 percent to 82 percent by 2009 (Greenspon, 2012). Several trials and evidence reviews on single and dual chamber pacemakers have been completed and reported since the NCD for cardiac pacemakers was originally established. Professional society guidelines have also been presented and revised over the years.
III. History of Medicare Coverage
Section 20.8 of the Medicare National Coverage Determinations (NCD) Manual is a long standing policy that dates back to 1983. This NCD establishes conditions of coverage and non-coverage for permanent single chamber and dual chamber cardiac pacemakers. In June 2000, Medtronic requested that CMS review the use of cardiac pacemakers to treat asymptomatic bradycardia in post-MI patients about to initiate long-term ß-blocker drug therapy. After a complete systematic review of the evidence provided, CMS posted a coverage decision memorandum (CAG-00063N) on March 20, 2001, that maintained the non-coverage NCD that pacemaker implantation would not be considered reasonable and necessary in these patients.
The NCD was last updated in 2004 when CMS issued a decision memorandum to focus the NCD on the indications for pacemaker use rather than on the pacemaker implantation procedure. This reflected CMS’ finding that pacemaker implantation was no longer an experimental procedure. CMS only changed the framework of the NCD and did not review the specific provisions setting forth conditions that indicate that cardiac pacing is reasonable and necessary.
A. Current Reconsideration
CMS received a formal external joint request from HRS and the American College of Cardiology (ACC) for reconsideration of dual-chamber pacemaker coverage. The request outlined differences between clinical guidelines and current Medicare coverage policy, focusing on revisions to the clinical indications for dual-chamber pacemakers. The requestor believes that the HRS/American College of Cardiology Foundation (ACCF) Expert Consensus Statement on Pacemaker Device and Mode Selection and additional evidence support expansion and clarification of Medicare coverage policy for the use of dual-chamber cardiac pacemakers.
B. Benefit Category
Medicare is a defined benefit program. An item or service must fall within a benefit category as a prerequisite to Medicare coverage [§1812 (Scope of Part A); §1832 (Scope of Part B); §1861(s) (Definition of Medical and Other Health Services)]. An item or service must meet one of the statutorily defined benefit categories in the Social Security Act and not otherwise be excluded. Cardiac pacemakers qualify as:
- Inpatient hospital services.
- Physicians’ services.
- Prosthetic devices.
Thus, cardiac pacemakers qualify as a benefit.
Note: This may not be an exhaustive list of all applicable Medicare benefit categories for this item or service.
IV. Timeline of Recent Activities
| Date |
Action |
| January 24, 2013 |
CMS initiates this national coverage analysis for reconsideration of Section 20.8 of the NCD Manual. The initial 30-day public comment period began with this posting date. |
| February 23, 2013 |
The initial 30-day public comment period ended. CMS received 98 comments. |
| May 29, 2013 |
CMS posted the proposed decision memorandum. The 30-day public comment period began with this posting date. |
| June 28, 2013 |
The 30-day public comment period ended. CMS received 50 comments.
|
V. Food and Drug Administration (FDA) Status
The class to which a device is assigned determines, among other things, the type of premarketing submission/application required for FDA clearance to market. If a device is classified as Class I or II, and if it is not exempt, a 510k will be required for marketing. For Class III devices, a premarket approval application (PMA) is required for marketing. Pacemakers that are currently developed which are considered similar to predicate devices are cleared through the 510k process. The FDA approves pacemakers for sino-atrial node dysfunction (e.g. sinus bradycardia, sinus arrest) and AV node dysfunction including second or third degree AV node block.
VI. General Methodological Principles
When making national coverage determinations under §1862(a)(1)(A), CMS generally evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that an item or service falling within a benefit category is reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member. The critical appraisal of the evidence enables us to determine to what degree we are confident that: 1) the specific assessment questions can be answered conclusively; and 2) the intervention will improve health outcomes for patients. An improved health outcome is one of several considerations in determining whether an item or service is reasonable and necessary.
A detailed account of the methodological principles of study design that the agency utilizes to assess the relevant literature on a therapeutic or diagnostic item or service for specific conditions can be found in Appendix A. In general, features of clinical studies that improve quality and decrease bias include the selection of a clinically relevant cohort, the consistent use of a single good reference standard, the blinding of readers of the index test, and reference test results. Public comments sometimes cite the published clinical evidence and give CMS useful information. Public comments that give information on unpublished evidence such as the results of individual practitioners or patients are less rigorous and therefore less useful for making a coverage determination. Public comments that contain personal health information (PHI) will be redacted and the PHI will not be made available to the public. CMS uses the initial public comments to inform its proposed decision. CMS responds in detail to the public comments on a proposed decision when issuing the final decision memorandum.
VII. Evidence
A. Introduction
In this coverage analysis, we considered evidence for single or dual chamber permanent cardiac pacemakers for the treatment of symptomatic bradycardia. Important health outcomes include mortality, morbidity (including adverse events), and quality of life (QOL). Increased survival has been demonstrated with initial use of single chamber pacemakers. The first patient to receive an implanted pacemaker was able to leave the hospital and lead a moderately active life for more than two years after receiving the implant. Given the past research and evidence on survival from use of permanent pacemakers, our analysis focuses on mortality, but also emphasizes other health outcomes. We note that while quality of life is an important health outcome, it is more difficult to objectively measure and discern potential bias in the reported studies.
B. Literature Search
CMS searched PubMed from February 2004 (date of the last NCD) to December 2012 using key words pacemakers, single and dual. We initially focused our search on randomized controlled trials (RCTs) and large prospective observational studies that evaluated adults ≥ 65 years. Observational studies with sample sizes less than 50 cases were excluded as they may have inherent biases that substantially limit generalizability. Abstracts, presentations, and articles not written in English were also excluded. In addition to our search, the requestors submitted 100 article summaries which were reviewed; seventeen articles met our criteria and were included in our review (five randomized trials, one randomized controlled comparison, one technology appraisal, one meta-analysis, two evidence reviews, one follow-up analysis of a randomized trial, one registry analysis, one secondary outcome analysis, three evidence-based guidelines, and one consensus statement).
CMS does not consider cost when developing NCDs under §1862(a)(1)(A). Some of the studies included in our review evaluated cost-effectiveness; however, CMS’ analysis did not rely on cost-effectiveness, nor did any cost effectiveness information impact the decision.
C. Discussion of Evidence Reviewed
Question:
Is the evidence sufficient to determine that implantation and use of permanent single chamber or dual chamber cardiac pacemakers improve health outcomes in Medicare beneficiaries?
1. External technology assessment
Castelnuovo E, Stein K, Pitt M, Garside R, Payne E. The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation. Health Technol Assess 2005;9(43).
Castelnuovo and colleagues reported the results of an evidence review (UK HTA: United Kingdom health technology assessment) “to estimate the effectiveness and cost effectiveness of dual-chamber pacemakers versus single-chamber atrial or single-chamber ventricular pacemakers in the treatment of bradycardia due to sick sinus syndrome (SSS) or atrioventricular block (AVB).” Patients with a primary diagnosis of acquired symptomatic bradycardia, secondary to SSS, AVB, or chronic bifascicular block, and individuals with symptomatic bradycardia in secondary and tertiary care centers were included. Eligible studies were systematic reviews and randomized trials. Non-randomized studies, case control, and cohort studies were excluded. Outcomes were mortality (all-cause and cardiovascular), stroke, atrial fibrillation (AF), heart failure, exercise capacity, symptoms of breathlessness, fatigue, chest pain, dizziness, palpitations, sleep disturbance, functional status, QOL, adverse events of implantation (perioperative mortality and non-fatal complications), and pacemaker syndrome.
Two evidence reviews, four parallel group randomized controlled trials (RCTs) (the Canadian Trial of Physiologic Pacing (CTOPP), the Mode Selection Trial (MOST), the Pacemaker Selection in the Elderly (PASE), and United Kingdom Pacing and Cardiovascular Events (UKPACE)) and 28 cross-over trials were included in the evidence review. The authors reported: “Dual chamber pacing was associated with lower rates of atrial fibrillation, particularly in SSS, than ventricular pacing, and prevents pacemaker syndrome. Higher rates of atrial fibrillation were seen with dual-chamber pacing than with atrial pacing. Complications occurred more frequently in dual-chamber pacemaker insertion.” They concluded: “Dual-chamber pacing results in small but potentially important benefits in populations with SSS and/or AVB compared with ventricular pacemakers.”
Dretzke J, Toff WD, Lip GYH, Raftery J, Fry-Smith A, Taylor RRS. Dual chamber versus single chamber ventricular pacemakers for sick sinus syndrome and atrioventricular block. Cochrane Database of Systematic Reviews 2004, Issue 2. Art. No.:CD003710. DOI: 10.1002/14651858.CD003710.pub2.
Dretzke and colleagues reported the results of an evidence review (the Cochrane review) “to assess the short- and long-term clinical effectiveness of dual chamber pacemakers compared to single chamber ventricular pacemakers in adults with AV block, sick sinus syndrome or both.” Patients 18 years and older with SSS and/or AVB were included. Eligible studies were “randomised controlled trials of either parallel group or crossover design comparing single chamber ventricular pacing with dual chamber pacing, or single chamber atrial pacing with dual chamber pacing.” Primary outcomes were cardiovascular mortality or all-cause mortality and cardiovascular morbidity (pacemaker syndrome, AF, stroke or heart failure).
Up to year 2002, five parallel (CTOPP, MOST, PASE, 1 trial on tachyarrhythmia, 1 trial on AF) and 26 cross-over randomized controlled trials were included in the review. The authors noted that “quality of reporting was found to be poor” and reported: “Pooled data from parallel studies shows a statistically non-significant preference for physiologic pacing (primarily dual chamber pacing) for the prevention of stroke, heart failure and mortality, and a statistically significant beneficial effect regarding the prevention of atrial fibrillation (odds ratio (OR) 0.79, 95% CI 0.68 to 0.93). Both parallel and crossover studies favour dual chamber pacing with regard to pacemaker syndrome (parallel: Peto OR 0.11, 95% CI 0.08 to 0.14; crossover: standardised mean difference (SMD) -0.74, 95% CI -0.95 to -0.52). Pooled data from crossover studies shows a statistically significant trend towards dual chamber pacing being more favourable in terms of exercise capacity (SMD -0.24, 95% CI -0.03 to -0.45). No individual studies reported a significantly more favourable outcome with single chamber ventricular pacing.” They concluded: “This review shows a trend towards greater effectiveness with dual chamber pacing compared to single chamber ventricular pacing, which supports the current British Pacing and Electrophysiology Group’s Guidelines regarding atrioventricular block. Additional randomised controlled trial evidence from ongoing trials in this area will further inform the debate.”
Grenouilleau-Albertini A, Copie X, Mabo P, et al. Single or dual-chamber pacemakers? The guidelines of the HAS. Archives of Cardiovascular Diseases Supplements. 2010; 2: 61-74.
Grenouilleau-Albertini and colleagues (HAS: Haute Autorité de Sandé (French: High Authority for Health)) reported the results of a technology appraisal “to assess single and dual-chamber pacemakers with a view to updating their reimbursement conditions by French National Health Insurance.” They analyzed “1 health technology assessment, 1 meta-analysis, 3 guidelines and 3 randomised controlled trials.” Inclusion and exclusion criteria were not reported. They found: “Pacing mode did not reduce mortality or heart failure hospitalizations. Compared with ventricular pacing, atrial-based pacing (physiologic modes) reduced stroke and atrial fibrillation (“AF”). There was a significant reduction in the composite outcome of stroke or cardiovascular mortality, but only among patients with SSS. Results of QOL, exercise capacity, functional status and pacemaker syndrome are variable.” They concluded: “HAS recommends generally implanting a single chamber pacemaker for the management of bradycardia. Dual-chamber pacemakers (DDDR) have to be reserved for 2 situations: (i) SSS with evidence of impaired atrioventricular conduction, (ii) permanent AVB without AF. HAS will not recommend that pacemakers with specific algorithms for minimal ventricular pacing be systematically used instead of single-chamber pacemakers in patients presenting with SSS or paroxysmal AVB, unless more clinical trials are performed.”
Healey JS, Toff WD, Lamas GA, Andersen HR, Thorpe KE, Ellenbogen KA, Lee KL, Skene AM, Schron EB, Skehan JD, Goldman L, Roberts RS, Camm AJ, Yusuf S, Connolly SJ. Cardiovascular outcomes with atrial-based pacing compared with ventricular pacing: meta-analysis of randomized trials, using individual patient data. Circulation. 2006 Jul 4;114(1):11-7. Epub 2006 Jun 26.
Healey and colleagues reported the results of a meta-analysis “to determine whether atrial-based pacing prevents major cardiovascular events” compared to ventricular pacing. Randomized trials from 1990 were included. Trials on multisite pacing and follow-up less than six months were excluded. Outcomes were clinical events, mortality, stroke, heart failure, and AF. Of the eight trials that met inclusion criteria, five trials had available patient level data and were included in the analysis (n = 758 patients with average follow-up of two years; 35000 patient years of follow-up). Mean age of participants in the trials ranged from 73 to 80 years. The authors reported: “There was no significant reduction in mortality (hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.87 to 1.03; P 0.19) or heart failure (HR, 0.89; 95% CI, 0.77 to 1.03; P 0.15) with atrial-based pacing. There was a significant reduction in atrial fibrillation (HR, 0.80; 95% CI, 0.72 to 0.89; P 0.00003) and a reduction in stroke that was of borderline significance (HR, 0.81; 95% CI, 0.67 to 0.99; P 0.035).” They concluded: “Compared with ventricular pacing, the use of atrial-based pacing does not improve survival or reduce heart failure or cardiovascular death. However, atrial-based pacing reduces the incidence of atrial fibrillation and may modestly reduce stroke.”
2. Internal technology assessment
Connolly SJ, Kerr CR, Gent M, Roberts RS, Yusuf S, Gillis AM, Sami MH, Talajic M, Tang AS, Klein GJ, Lau C, Newman DM. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physiologic Pacing Investigators. N Engl J Med. 2000 May 11;342(19):1385-91.
Connolly and colleagues reported the results of the Canadian Trial of Physiologic Pacing (CTOPP), a randomized trial that evaluated “the benefit of physiologic pacing in patients requiring a pacemaker for symptomatic bradycardia.” Patients were eligible if they were “scheduled for an initial implantation of a pacemaker to correct symptomatic bradycardia, did not have chronic atrial fibrillation, and were at least 18 years old.” Exclusion criteria were atrioventricular nodal ablation or life expectancy less than two years. Primary outcome was the occurrence of either stroke or death due to cardiovascular causes. Secondary outcomes were death from any cause, documented AF, and congestive heart failure hospitalization. A total of 1474 patients were randomly assigned to receive a ventricular pacemaker and 1094 to a physiologic pacemaker (dual-chamber, atrial and ventricular). Mean age at baseline was 73 years. Men comprised 59 percent of the study population. AVB was the most common indication for pacing. Sinoatrial node disease was present in 40 percent of patients. Mean follow-up was three years.
The authors reported: “The annual rate of stroke or death due to cardiovascular causes was 5.5 percent with ventricular pacing, as compared with 4.9 percent with physiologic pacing (reduction in relative risk, 9.4 percent; 95 percent confidence interval, –10.5 to 25.7 percent [the negative value indicates an increase in risk]; P = 0.33).” They concluded: “Physiologic pacing provides little benefit over ventricular pacing for the prevention of stroke or death due to cardiovascular causes.”
Fleishmann KE, Orv EJ, Lamas GA, Mangione CM, Schron EB, Lee KL, Goldman I.: MOST investigators. Atrial fibrillation and quality of life after pacemaker implantation for sick sinus syndrome; data from the Mode Selection Trial (MOST). Am Heart J. 2009 Jul;158(1):78-83.e2.doi: 10.1016/j.ahj.2009.02.023.
Fleischmann and colleagues reported the results of a specific analysis of QOL as a pre-specified secondary outcome of the Mode Selection Trial (MOST; see primary results reported by Lamas, 2002). QOL was measured with the Medical Outcomes Study 36-Item Short Form (SF-36) General Health Survey at baseline, three months, 12 months, 24 months, 36 months, and 48 months. The investigators found that “[p]acemaker implantation resulted in substantial improvement in almost all QOL measures” but “[s]ubjects 75 years or older experienced significantly less improvement in functional status and physical component summary scores than did younger subjects.” They concluded: “Pacemaker implantation improved health-related QOL. The mode selected was associated with much smaller, but significant, improvements in several domains, particularly role physical function.”
Fored CM, Granath F, Gadler F, Blomqvist P, Rynder J, Linde C, Ekbom A, Rosenqvist M. Atrial vs. dual-chamber cardiac pacing in sinus node disease: a register-based cohort study. Europace. 2008 Jul;10(7):825-31. doi: 10.1093/europace/eun118. Epub 2008 May 7.
Fored and colleagues reported the results of an analysis of a pacemaker registry “to compare the incidence of cardiovascular morbidity and mortality in patients with sinus node disease.” Patients who had sinus node disease and received an AAI- or DDD-mode pacemaker implant between 1989 and 2002 and were identified from the Swedish implantable cardioverter defibrillator and pacemaker register (n = 9563). Patients with “a hospital discharge diagnosis of stroke prior to the pacemaker implant were excluded (n = 786).” Primary outcomes were the first hospitalization for, or death from AF or flutter, stroke, or any cardiovascular disease. Of the 8777 patients in the analysis, 2685 received atrial pacing (AAI; 30.6%) and 6092 received dual chamber pacing (DDD; 69.4%). Eighty-seven percent of the patients were 60 years or older. Men comprised 47 percent of the cohort. Mean follow-up was four years for AAI patients and 2.8 years for DDD patients.
The authors reported: “Patients with DDD pacing and without any pre-implant admission for atrial fibrillation or flutter had an increased risk of post-implant fibrillation or flutter, in relation to corresponding AA[I] patients [hazard ratio (HR) = 1.30; 95% confidence interval (CI) 1.10–1.52]. A slight increase in the risk of any cardiovascular disease (HR = 1.07; CI, 1.00–1.15), and all-cause mortality (HR = 1.12; CI, 1.00–1.25), was seen among DDD patients, in relation to AAI patients, but there was no significant difference in the risk of ischaemic or unspecified stroke (HR = 1.14; CI, 0.94–1.37). Among DDD patients, the all-cause mortality did not differ from the general population [standardized mortality ratio (SMR) = 1.04; CI, 0.98–1.11]. Patients with AAI, however, had a decreased all-cause mortality risk (SMR = 0.89; CI, 0.82–0.97).” They concluded: “Our results support AAI as the preferred mode of pacing in patients with sinus node disease, and a normal AV node function.”
Kerr CR, Connolly SJ, Abdollah H, Roberts RS, Gent M, Yusuf S, Gillis AM, Tang AS, Talajic M, Klein GJ, Newman DM. Canadian Trial of Physiological Pacing: Effects of physiological pacing during long-term follow-up. Circulation. 2004 Jan 27;109(3):357-62. Epub 2004 Jan 5.
Kerr and colleagues reported the results of an extended follow-up analysis of CTOPP, a randomized trial that compared “physiological pacing that maintained atrioventricular synchrony (dual-chamber or atrial pacing) compared with ventricular pacing with regard to the composite outcome of cardiovascular death and stroke,” reported by Connolly in 2000. Of the 2568 patients randomized in CTOPP, 1995 were eligible for the extended follow-up study with 94.9 percent follow-up at three years. The primary outcome was the composite of cardiovascular death or stroke. Secondary outcomes were overall mortality, stroke, and new development of AF. Mean follow-up of the combined CTOPP and extended study was 6.4 years. The authors concluded: “The CTOPP extended study does not show a difference in cardiovascular death or stroke, or in total mortality, or in stroke between patients implanted with ventricular or physiological pacemakers over a mean follow-up of 6 years. However, there is a persistent significant reduction in the development of atrial fibrillation with physiological pacing.”
Lamas GA, Lee KL, Sweeney MO, Silverman R, Leon A, Yee R, Marinchak RA, Flaker G, Schron E, Orav EJ, Helkamp AS, Greer S, McAnulty J, Elenbogen K, Ehlert F, Freeman RA, Estes NA 3rd, Greenspon A, Goldman L: Mode Selection Trial in Sinus-Node Dysfunction. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med. 2002 Jun 13:346(24):1854-62.
Lamas and colleagues reported the results of a randomized trial to determine “whether dual-chamber pacing would provide better event-free survival and quality of life than single chamber ventricular pacing in patients with sinus-node dysfunction.” Patients aged 21 years and older with SND in sinus rhythm were eligible. Patients with serious comorbidities (study site determined) were excluded. Primary outcome was death from any cause or nonfatal stroke. Prespecified secondary outcomes included cardiovascular mortality, AF and QOL.
From September 1995 to October 1999, 2010 patients were enrolled at 91 sites. All patients received a dual chamber, rate modulated pacemaker but the device programming varied with assignment. Patients were randomly assigned to dual chamber pacing (n = 1014) or ventricular pacing (n = 996). Median age was 74 years. Men comprised 52 percent of the study population. Median follow-up was 33.1 months. The investigators found that “incidence of the primary end point did not differ significantly between the dual-chamber group (21.5 percent) and the ventricular-paced group (23.0 percent, P = 0.48).” They concluded: “In sinus-node dysfunction, dual-chamber pacing does not improve stroke-free survival, as compared with ventricular pacing. However, dual chamber pacing reduces the risk of atrial fibrillation, reduces signs and symptoms of heart failure, and slightly improves the quality of life. Overall, dual chamber pacing offers significant improvement as compared with ventricular pacing.”
Lamas GA, Orav EJ, Stambler BS, Ellenbogen KA, Sgarbossa EB, Huang SK, Marinchak RA, Estes NA 3rd, Mitchell GF, Lieberman EH, Mangione CM, Goldman L. Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing. Pacemaker Selection in the Elderly Investigators. N Engl J Med. 1998 Apr 16;338(16):1097-104.
Lamas and colleagues reported the results of a randomized, controlled comparison of ventricular pacing and dual chamber pacing to assess the effect of the pacing mode on the health-related QOL of older adults. Inclusion criteria were age of 65 years or older, sinus rhythm, and a permanent pacemaker requirement for bradycardia. Exclusion criteria were inability to participate in the QOL assessments, clinically overt congestive heart failure at the time of implantation, AF without any documented sinus mechanism for more than six months, serious non-cardiac illness, and inadequate atrial-capture or sensing thresholds. Primary outcome was multidimensional health-related QOL measured with the 36-item Medical Outcomes Study Short-Form General Health Survey (SF-36) at three, nine and 18 months. Pre-specified secondary end points were “death from all causes; first nonfatal stroke or death; first hospitalization for heart failure, first nonfatal stroke, or death; development of atrial fibrillation; and development of the pacemaker syndrome.”
From February 1993 to September 1994, 407 patients were enrolled at 29 sites. All patients received dual chamber rate-adaptive pacemakers, but the device programming varied with assignment. Patients were randomly assigned to single chamber ventricular pacing (n = 204) or dual chamber pacing (n = 203). Mean age was 76 years. Men comprised 60 percent of the study population. The authors reported: “Quality of life improved significantly after pacemaker implantation (P < 0.001), but there were no differences between the two pacing modes in either the quality of life or prespecified clinical outcomes (including cardiovascular events or death).” They concluded: “The implantation of a permanent pacemaker improves health-related quality of life. The quality-of-life benefits associated with dual-chamber pacing as compared with ventricular pacing are observed principally in the subgroup of patients with sinus-node dysfunction.”
Nielsen JC, Kristensen L, Andersen HR, Mortensen PT, Pedersen OL, Pedersen AK. A randomized comparison of atrial and dual-chamber pacing in 177 consecutive patients with sick sinus syndrome: echocardiographic and clinical outcome. J Am Coll Cardiol. 2003 Aug 20;42(4):614-23.
Nielsen and colleagues reported the results of a randomized trial “to compare single-chamber atrial (AAI) and dual-chamber (DDD) pacing in patients with sick sinus syndrome (SSS).” Inclusion criteria were symptomatic bradycardia < 40 bpm or symptomatic QRS pauses of more than two seconds. Exclusion criteria included AVB, chronic AF and bundle branch block. Primary outcomes were “changes in left atrial (LA) size and left ventricular (LV) size and function as measured by M-mode echocardiography.” Secondary outcomes included death, stroke and AF. From December 1994 to March 1999, 177 consecutive patients were randomized to one of three rate-adaptive pacemakers: single chamber atrial (AAIR; n = 54), dual chamber (DDDR-s with a short AV delay; n = 60) or dual chamber (DDDR-l with a fixed long AV delay; n = 63). Mean age was 75 years. Men comprised 41 percent of the study population. Mean follow-up was three years.
The authors reported: “DDDR pacing causes increased LA diameter, and DDDR pacing with a short atrioventricular delay also causes decreased LVFS [left ventricular fractional shortening]. No changes occur in LA or LV diameters or LVFS during AAIR pacing. Atrial fibrillation is significantly less common during AAIR pacing.” No significant differences in mortality, stroke and heart failure were detected between groups.
Nielsen JC, Thomsen PE, Højberg S, Møller M, Vesterlund T, Dalsgaard D, Mortensen LS, Nielsen T, Asklund M, Friis EV, Christensen PD, Simonsen EH, Eriksen UH, Jensen GV, Svendsen JH, Toff WD, Healey JS, Andersen HR; DANPACE Investigators. A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur Heart J. 2011 Mar;32(6):686-96. doi: 10.1093/eurheartj/ehr022. Epub 2011 Feb 7.
Nielsen and colleagues reported the results of a randomized trial to compare single lead atrial pacing (AAIR) and dual chamber pacing (DDDR) in patients with SSS (DANPACE: The Danish Multicenter Randomized Trial on Single Lead Atrial Pacing versus Dual Chamber Pacing in Sick Sinus Syndrome). Inclusion criteria were symptomatic bradycardia, documented sino-atrial block or sinus-arrest with pauses > 2 seconds or sinus bradycardia < 40 bpm for more than one minute while awake, PR interval ≤ 0.22 seconds if aged 18–70 years or PR interval ≤ 0.26 seconds if aged ≥ 70 years and QRS width < 0.12 seconds. Exclusion criteria included AVB, bundle branch block, long-standing persistent AF (> 12 months), AF with ventricular rate < 40 bpm for ≤ 1 minute or pauses > 3 seconds, a positive test for carotid sinus hypersensitivity, planned cardiac surgery, or a life-expectancy shorter than one year. Primary outcome was death from any cause. Secondary outcomes included paroxysmal AF, chronic AF, stroke, peripheral embolism, heart failure and pacemaker re-operation. From March 1999 to June 2008, 1415 patients were randomly assigned to AAIR (n = 707) or DDDR (n = 708). Mean age was 73 years. Men comprised 35 percent of the study population. Mean follow-up was five years.
The authors did not detect significant differences in death (29.6% vs. 27.3%), chronic AF (11.2% vs. 10.7%), stroke (5.5% vs. 4.8%) or heart failure. They concluded: “In patients with sick sinus syndrome, there is no statistically significant difference in death from any cause between AAIR pacing and DDDR pacing. AAIR pacing is associated with a higher incidence of paroxysmal atrial fibrillation and a two-fold increased risk of pacemaker reoperation. These findings support the routine use of DDDR pacing in these patients.”
Toff WD, Camm AJ, Skehan JD; United Kingdom Pacing and Cardiovascular Events Trial Investigators. Single-chamber versus dual-chamber pacing for high-grade atrioventricular block. N Engl J Med. 2005 Jul 14;353(2):145-55.
Toff and colleagues reported the results of a randomized trial to compare the clinical benefits of single chamber ventricular pacing and dual chamber pacing in elderly patients with AVB. Patients were 70 years of age or older and were scheduled to receive their first pacemakers for high-grade (second-degree or complete) AVB. Exclusion criteria were “chronic established atrial fibrillation, or newly diagnosed ischemic heart disease, and myocardial infarction.” Primary outcome was death from all causes. Secondary outcomes included “atrial fibrillation (defined as an episode, with or without symptoms, lasting 15 minutes or more and verified by electrocardiography), new or significantly worsening heart failure, a composite of stroke, transient ischemic attack, or other thromboembolism, revision of the pacing system, new-onset angina or newly diagnosed ischemic heart disease, and myocardial infarction.”
From August 1995 to September 1999, 2021 patients were enrolled and randomly assigned to single-chamber ventricular pacing (fixed rate: n = 504 and adaptive: n = 505) and to dual-chamber pacing (n = 1012). Mean age was 80 years. Men comprised 57 percent of the study population. Mean follow-up was “4.6 years for death and 3 years for other cardiovascular events.” The authors reported: “The mean annual mortality rate was 7.2 percent in the single-chamber group and 7.4 percent in the dual-chamber group (hazard ratio, 0.96; 95 percent confidence interval, 0.83 to 1.11). We found no significant differences between the group with single-chamber pacing and that with dual-chamber pacing in the rates of atrial fibrillation, heart failure, or a composite of stroke, transient ischemic attack, or other thromboembolism.” They concluded: “In elderly patients with high-grade atrioventricular block, the pacing mode does not influence the rate of death from all causes during the first five years or the incidence of cardiovascular events during the first three years after implantation of a pacemaker.”
3. Medicare Evidence Development & Coverage Advisory Committee (MEDCAC)
The MEDCAC was not convened for this review.
4. Evidence-based guidelines
Epstein AE, Dimarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 Guidelines for device-based therapy of cardiac rhythm abnormalities. Heart Rhythm. 2008 Jun;5(6):e1-62. doi: 10.1016/j.hrthm.2008.04.014. Epub 2008 May 21.
Epstein AE, DiMarco JP, Ellenbogen KA, Estes NAM III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices). Heart Rhythm 2008;5:e1–e62.
In 2008, ACC, AHA, and HRS published evidence-based guidelines on device-based therapy for arrhythmias. Recommendations were reported for arrhythmias as well as device selection. Table 2 (reproduced below) presented indications for single chamber and dual chamber pacemakers.

National Institute for Clinical Excellence. Dual-chamber pacemakers for symptomatic bradycardia due to sick sinus syndrome and/or atrioventricular block. Technology Assessment 88. February 2005.
The National Institute for Clinical Excellence (NICE) published a technology appraisal with evidence-based guidelines on dual chamber pacemakers for symptomatic bradycardia due to SSS and/or AVB. A total of 32 studies of
dual chamber pacemakers versus single chamber ventricular pacemakers were identified. “Four parallel randomised controlled trials (RCTs) allocated a total of 7006 patients to single- or dual-pacing modes (MOST and PASE studies), or single- or dual-chamber devices (CTOPP and the unpublished UKPACE study).” The Institute noted: “Dual-chamber pacing is recommended for the management of symptomatic bradycardia due to sick sinus syndrome, atrioventricular block, or
a combination of sick sinus syndrome and atrioventricular block, except:
- in the management of sick sinus syndrome in patients in whom, after full evaluation, there is no evidence of impaired atrioventricular conduction; in this situation, single-chamber atrial pacing is appropriate
- in the management of atrioventricular block in patients with continuous atrial fibrillation; in this situation, single-chamber ventricular pacing is appropriate
- in the management of atrioventricular block (atrioventricular block alone, or in combination with sick sinus syndrome), when patient-specific factors, such as frailty or the presence of comorbidities, influence the balance of risks and benefits in favour of single-chamber ventricular pacing.”
Vardas PE, Auricchio A, Blanc JJ, Daubert JC, Drexler H, Ector H, Gasparini M, Linde C, Morgado FB, Oto A, Sutton R, Trusz-Gluza M; European Society of Cardiology; European Heart Rhythm Association. Guidelines for cardiac pacing and cardiac resynchronization therapy: The Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Eur Heart J. 2007 Sep;28(18):2256-95. Epub 2007 Aug 28.
The European Society of Cardiology and European Heart Rhythm Association jointly published guidelines for cardiac pacing. For sinus node disease, the recommendations are below (copy of Table 1.1.1):

For AVB, the recommendations are below (copy of Table 1.2.1):
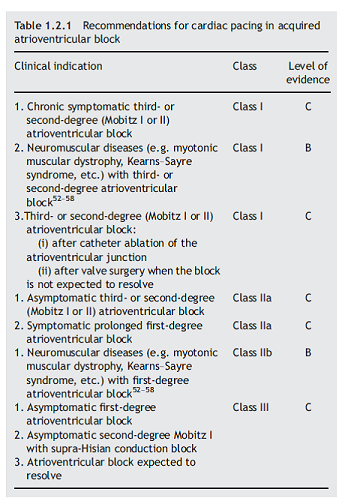
5. Consensus Statement
Gillis AM, Russo AM, Ellenbogen KA, Swerdlow CD, Olshansky B, Al-Khatib SM, Beshai JF, McComb JM, Nielsen JC, Philpott JM, Shen WK; Heart Rhythm Society; American College of Cardiology Foundation, HRS/ACCF expert consensus statement on pacemaker device and mode selection. Developed in partnership between the Heart Rhythm Society (HRS) and the American College of Cardiology Foundation (ACCF) and in collaboration with the Society of Thoracic Surgeons, Heart Rhythm, 2012 Aug;9(8):1344-65, doi: 10.1016/j.hrthm.2012.06.026.
Gillis AM, Russo AM, Ellenbogen KA, Swerdlow CD, Olshansky B, Al-Khatib SM, Beshai JF, McComb JM, Nielsen JC, Philpott JM, Shen WK. HRS/ACCF expert consensus statement on pacemaker device and mode selection. J Am Coll Cardiol. 2012 Aug 14:60(7):682-703. Doi:10.1016/j.jacc.2012.06.011. Epub 2012 Jul 30.
Gillis and colleagues reported the opinions of the HRS/ACCF consensus writing group on pacemaker device and mode selection. The authors noted that “recommendations for device selection in the current document apply to situations where the clinical decision for pacing has already been made.” The Class I (see Appendix F - “Conditions for which there is evidence and/or general agreement that a given pacing mode is beneficial, useful and effective.”) recommendations were:
- “Dual-chamber pacing (DDD) or single-chamber atrial pacing (AAI) is recommended over single-chamber ventricular pacing (VVI) in patients with SND and intact AV conduction (Level of Evidence: A).”
- “Dual-chamber pacing is recommended over single-chamber atrial pacing in patients with SND (Level of Evidence: B).”
- “Dual-chamber pacing is recommended in patients with AV block (Level of Evidence: C).”
- “Single-chamber ventricular pacing is recommended as an acceptable alternative to dual-chamber pacing in patients with AV block who have specific clinical situations that limit the benefits of dual-chamber pacing. These include, but are not limited to, sedentary patients, those with significant medical comorbidities likely to impact clinical outcomes, and those in whom technical issues, such as vascular access limitations, preclude or increase the risk of placing an atrial lead (Level of Evidence: B).”
- “Dual-chamber pacing is recommended over single-chamber ventricular pacing in adult patients with AV block who have documented pacemaker syndrome (Level of Evidence: B).”
6. Public Comments
The comments can be viewed in their entirety on our website at http://www.cms.gov/medicare-coverage-database/details/nca-view-public-comments.aspx?NCAId=267&ExpandComments=n&NcaName=Cardiac+Pacemakers*3a%24+Single-Chamber+and+Dual-Chamber+Permanent+Cardiac+Pacemakers+(3rd+Recon)&bc=AiAAAAAACAAAAA%3d%3d&.
Initial 30-day Comment Period
During the initial 30-day comment period, CMS received 98 comments from various entities including: the American Heart Association/American Stroke Association, the Heart Rhythm Society, device manufacturers, coding consultants, hospital health systems, physicians, health network utilization review departments, hospitals, case managers, and the general public.
Ninety-four (94) comments supported modification of Medicare coverage of dual chamber pacemakers, of which 80 comments supported modifications consistent with the opinions of the HRS/ACCF consensus writing group. Of the 80 comments, 42 were form letter submissions. Five comments also included suggested changes for coverage of single chamber pacemakers.
One commenter did not support modification of the current NCD. Three comments did not express a position regarding modification of the NCD, but raised issues about coding and enforcement of existing policy.
Public Comments on the Proposed Decision Memorandum
We received 50 comments on the proposed decision from various entities including physician specialty societies, hospitals, health networks, physicians, community health networks, a health policy association, device manufacturers, and the general public. Forty-nine (49) comments supported the proposed decision, with nine of those comments suggesting modifications to the proposed covered and non-covered indications. One comment opposed the proposed decision. Three comments included references to guidelines, consensus statements, and articles that were previously included in our review of the evidence. Among the three comments that furnished references, one included a reference to the NCD for implantable cardiac defibrillators, which is outside the scope of this analysis. Another commenter included references to general clinical articles on medical management, which are not considered primary clinical research studies. The same commenter referenced articles that address topics that are outside the scope of this national coverage analysis. Specific issues and suggested modifications furnished by commenters are discussed below.
Comment:
Commenters opined that coverage was appropriate for patients with sinus node dysfunction and sick sinus syndrome.
Response:
In the background section of this decision memorandum we state that sinus node dysfunction is also known as sick sinus syndrome; we believe these terms to be synonymous. Based on the trials included in our analysis, we believe the evidence supports the use of permanent single chamber or dual chamber cardiac pacemakers for treatment of documented symptomatic bradycardia due to SND.
Comment:
Commenters requested coverage for asymptomatic second degree AVB of Mobitz Type I unless the QRS complexes are prolonged or electrophysiological studies have demonstrated that the block is at or beyond the level of the His Bundle (a component of the electrical conduction system of the heart).
Response:
In general, individuals without symptoms are not treated with permanent implantable single chamber or dual chamber pacemakers. The patients in the studies we evaluated were symptomatic. Patients with progressive heart block may develop symptoms in the future that could lead to a patient having a covered indication. Based on our review of the published evidence, we did not find specific evidence to support coverage for this indication. Therefore, we are finalizing non-coverage for this indication.
Comment:
Commenters requested coverage for prophylactic pacemaker use following recovery from acute myocardial infarction during which there was temporary complete (third-degree) and/or Mobitz Type II second-degree AVB in association with bundle branch block, which we proposed to non-cover.
Response:
We recognize that various arrhythmias may occur in patients with acute myocardial infarctions and that this indication has been covered for single chamber pacemakers under existing policy, meaning that patients may have received this treatment under certain circumstances. We are permitting local Medicare contractors to make the coverage determination under section 1862(a)(1)(A) for prophylactic pacemaker use following recovery from acute myocardial infarction during which there was temporary complete (third-degree) and/or Mobitz Type II second-degree AVB in association with bundle branch block.
Comment:
Commenters suggested that hypersensitive carotid sinus syndrome should be considered a covered indication based on consensus recommendations that stated permanent pacing is indicated for recurrent syncope caused by spontaneously occurring carotid sinus stimulation and carotid sinus pressure that induces ventricular asystole of more than three seconds (Class I Recommendation, Level of Evidence C); and permanent pacing is reasonable for syncope without clear, provocative events and with a hypersensitive cardioinhibitory response of three seconds or longer (Class IIa Recommendation, Level of Evidence C). Commenters also suggested that we remove the example from one of the non-covered indications since it references hypersensitive carotid sinus syndrome.
Response:
Our covered indications do not specifically address hypersensitive carotid sinus syndrome, thus we are permitting local Medicare contractors to make the coverage determination for this indication under section 1862(a)(1)(A). We are modifying one non-covered indication for clarity and to ensure there is internal consistency with our covered indications, to read as follows: “a clinical condition in which pacing takes place only intermittently and briefly, and which is not associated with a reasonable likelihood that pacing needs will become prolonged.”
Comment:
One commenter suggested that there was a technical error in our proposed non-coverage of P-R intervals with atrial fibrillation (without third-degree atrioventricular block).
Response:
We appreciate the commenter’s feedback. In patients with atrial fibrillation, the P-R interval may be difficult to detect and relate to bradycardia. In this final decision, we are permitting local Medicare contractors to make the coverage determination under section 1862(a)(1)(A) for this indication.
Comment:
One commenter suggested that patients with symptomatic reversible sinus bradycardia that results from required drug therapy for medical conditions should be covered.
Response:
Reversible causes of bradycardia (such as electrolyte abnormalities, medications or drugs, or hypothermia) are transient and not permanent in nature. The published evidence does not support permanent pacemaker implantation for
conditions that are transient or not permanent in nature. Therefore, we are not making the requested change.
Comment:
Some commenters requested that we allow coverage for the non-covered indications when evaluated in Category B IDE clinical trials.
Response:
Local contractors are bound by national coverage determinations in their review of claims for payment, including evaluations for coverage in Category B IDE clinical trials. Nationally non-covered indications would not be coverable by local contractors. Based on the evidence reviewed, we are not making the requested change in this NCD.
Comment:
Commenters identified the following indications that were not specifically addressed in the proposed decision, but believed these indications should be covered:
- Any form of brady-tachy syndrome;
- Pacemaker syndrome or indications that pacemaker syndrome may develop in the future;
- Asymptomatic second and third degree AVB;
- Asymptomatic Mobitz type II AVB or complete heart block, which are infrequently picked up on 24 hour monitoring or while a patient is in the hospital;
- Symptomatic or asymptomatic chronotropic incompetence, irrespective of whether a patient’s heart rate is above or below 60 bpm;
- Second degree Mobitz Type I AVB when QRS complexes are prolonged, regardless of the presence of symptoms;
- Patients in sinus rhythm that develop indications for heart block;
- Symptomatic or at high risk of congenital long QT syndrome; and
- Bifascicular or trifascicular block accompanied by syncope which is attributed to transient complete heart block after other plausible causes of syncope have been reasonably excluded.
Response:
Based on the trials included in our analysis, we believe the evidence supports the use of permanent single chamber or dual chamber cardiac pacemakers for treatment of documented non-reversible symptomatic bradycardia due to SND or second degree and/or third degree AVB.
Medicare Administrative Contractors will determine coverage under section 1862(a)(1)(A) of the Social Security Act for any other indications for the implantation and use of single chamber or dual chamber cardiac pacemakers that are not specifically addressed in this national coverage determination.
Comment:
Commenters requested coverage for patients with medically refractory symptomatic hypertrophic cardiomyopathy or provoked left ventricular outflow obstruction, and coverage for patients with recurrent and refractory ventricular tachycardia (overdrive pacing – pacing above the basal rate) to prevent ventricular tachycardia.
Response:
Overdrive pacing, generally considered to be related to the use of implanted cardiac defibrillators (ICDs) and treatment of medically refractory symptomatic hypertrophic cardiomyopathy were not considered. As discussed in the background section, the scope of this reconsideration and this coverage determination does not address biventricular pacemakers, pacemakers that stimulate more than two heart chambers, those devices used to treat tachyarrhythmias and cardiac dyssynchrony, cardiac resynchronization therapy, cardiac pacemaker evaluation services, or self-contained pacemaker monitors. Therefore, we believe these indications fall outside the scope of this national coverage analysis.
Comment:
Commenters disagreed with our assessment that dual chamber pacemakers are neither superior nor inferior to single chamber pacemakers and believe the statement should be deleted from the decision memorandum. Some suggested that we modify statements that address higher complication rates with dual chamber pacing.
Response:
Our statement was based on the trials included in our analysis that reported no difference in mortality, the primary outcome of interest, between single chamber and dual chamber cardiac pacemakers. As noted in the analysis, there is some indication of better secondary outcomes from use of dual chamber pacemakers compared to single chamber, although not uniformly significant. Health outcomes are comparable for patients who receive dual chamber pacemakers compared to those who receive single chamber pacemakers for symptomatic bradycardia but the choice of device may be influenced by the reduced rate of AF and higher perioperative complications seen with implantation of dual chamber pacemakers. We recognize the statement may be generally interpreted to apply to all outcomes whether primary or secondary and, therefore, have revised the analysis accordingly.
Comment:
Some commenters disagreed with the need to document and provide justification of therapeutic choice in the medical record, while other commenters requested guidance regarding the type of information that should be included in the patient’s medical record.
Response:
Shared decision making between the physician and patient remains important for device choice on an individual basis. We expect that physicians will carefully document the need for dual chamber pacemakers in the medical record, and that patients will be informed of the potentially higher complication rates following implantation of dual chamber devices as reported in the randomized controlled trials. The use of evidence-based guidelines and expert consensus statements may also help to inform the device selection process.
While pacemaker longevity has increased over the past few decades, replacement of a permanent cardiac pacemaker is occasionally needed due to wear or damage to one or more components (battery, generator, or leads). We did not find any specific evidence or recommendations on how to replace these devices or on which replacement devices should be used. Given the nature of the reasons for replacements, prudent clinical judgment should be used on an individual basis, along with documentation in the medical record on the initial indication for the pacemaker, the reason for replacement, and the justification of the type of replacement device selected. In rare instances, historical medical records may not be immediately available; however, adequate documentation should still be maintained to explain the existing circumstances and situation.
Practitioners may wish to contact their local Medicare Administrative Contractors for additional guidance regarding medical record documentation.
Comment:
The majority of commenters requested that this coverage determination be applied retroactively three to five years due to concerns about enforcement of existing coverage requirements.
Response:
This NCD is effective for claims with dates of service on or after the date of the NCD. NCDs are not applied retroactively.
VIII. CMS Analysis
National coverage determinations (NCDs) are determinations by the Secretary with respect to whether or not a particular item or service is covered nationally by Medicare (§1862(l) of the Social Security Act). In order to be covered by Medicare, an item or service must fall within one or more benefit categories contained within Part A or Part B, and must not be otherwise excluded from coverage. Moreover, section 1862(a)(1) of the Social Security Act in part states, with limited exceptions, no payment may be made under Part A or Part B for any expenses incurred for items or services, which are not reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member (§1862(a)(1)(A)).
As noted earlier, our review sought to answer the following question, which has been repeated here for the convenience of the reader.
Is the evidence sufficient to determine that implantation and use of permanent single or dual chamber cardiac pacemakers improve health outcomes in Medicare beneficiaries?
Since the first successful use of a permanent single chamber cardiac pacemaker in 1960 resulted in a post-implant survival of 18 months for a patient with complete heart block (Chardack, 1960; Beck, 2010), various studies and reviews (Edhag, 1976; Hansen, 1974; Müller, 1988; Rosenqvist, 1992) have supported the improvements in health outcomes from implantation and use of single chamber pacemakers. Hansen and Meibom (1974) reported one year survival of 87 percent for patients with complete heart block treated with permanent pacemakers compared to 50 percent for similar patients without pacemakers (Johansson, 1969). Rosenqvist and Norlander (1992) reported that “[i]n spite of lack of controlled studies it has been convincingly shown that VVI [fixed rate ventricular] pacing prolongs life in patients with high-grade AV block.” There is an established and well-accepted association of implantation and use of permanent single chamber pacemakers and improved health outcomes.
Over the past five decades, as medical knowledge and technology have advanced, the cardiac pacemaker has evolved from a bulky single lead device to a category of sophisticated devices with one or more leads capable of administering complex pacing programs and auto-monitoring. Greenspon (2012) noted the rapid uptake and utilization of these advanced devices in place of the single lead pacemaker, which is historically the recognized standard for symptomatic bradycardia. With the changes in the past decades, CMS has conducted this analysis to update the coverage decision from the early 1980s. Ideally there should be robust evidence on benefits of new technology prior to widespread adoption, to support clinically important advancements in the health of the population.
For symptomatic bradycardia, five randomized controlled trials (CTOPP, DANPACE, MOST, PASE, UKPACE), two systematic evidence reviews (Cochrane and UK HTA) and evidence-based guidelines from professional societies were included in our analysis for the health outcome of all-cause mortality (primary outcome in DANPACE and UKPACE; pre-specified secondary outcome in CTOPP, PASE, and MOST). None of the trials demonstrated significant differences in all-cause mortality between dual chamber pacemakers and single chamber pacemakers. In other words, the mortality rate in patients receiving dual chamber pacemakers was comparable to the rate in patients who received single chamber pacemakers for symptomatic bradycardia due to SND or AV conduction blocks. There were also no reported significant differences in stroke or cardiovascular death. While the Cochrane review was published in 2004 and did not include two trials (DANPACE, UKPACE), the findings were consistent with our review. The UK HTA appraisal (2005), which did not include DANPACE and the final published results of UKPACE, also found no significant effect on mortality. Although there are no suggestions that the reviews will be revised, it seems unlikely that the inclusion of the results of these two trials would markedly alter the conclusions of the Cochrane review and UK HTA appraisal given the reported
findings of DANPACE and UKPACE.
While there is no reported difference in mortality between single chamber and dual chamber cardiac pacemakers, there is some indication of better secondary outcomes from use of dual chamber pacemakers, including reduction in occurrence of AF and improved quality of life. For AF, two trials (CTOPP, MOST) found small but significant absolute reductions in rate of AF in patients receiving dual chamber pacemakers compared to single chamber. Connolly and colleagues (CTOPP) noted that “for every 100 patients treated for three years with physiologic rather than ventricular pacing, the occurrence of atrial fibrillation would be prevented in 4 patients.” Kerr (2004) reported that “there is a persistent significant reduction in the development of atrial fibrillation with physiological pacing” after analysis of six year follow-up data from CTOPP. Lamas (MOST) reported “when compared with ventricular pacing, dual-chamber pacing reduces newly diagnosed and chronic atrial fibrillation.” Nielsen (DANPACE) reported a difference in paroxysmal AF but not chronic AF, although the other trials did not make this fine differentiation. Toff (UKPACE) did not find any significant difference in AF.
Quality of life was a primary outcome in one study (PASE) and a secondary outcome in another (MOST). Lamas (PASE) reported that health related quality of life improved with permanent pacemaker implantation but there was no significant difference between single and dual chamber pacemakers. Similar results were reported by Fleischmann (MOST) who noted that “[p]acemaker implantation improved health-related QOL” but QOL subscale results were mixed according to pacemaker mode. Quality of life is an important patient-centered outcome but measurement of this outcome may be subject to participant and recall bias. Periprocedural complications (usually within 30 days of implantation) were specifically reported in two trials (CTOPP, UKPACE) and were significantly higher for dual chamber pacemakers compared to single chamber pacemakers.
Based on our review of the published evidence of these trial results, health outcomes are comparable for patients who receive dual chamber pacemakers compared to those who receive single chamber pacemakers for symptomatic bradycardia but the choice of device may be influenced by the reduced rate of AF and higher perioperative complications seen with implantation of dual chamber pacemakers. The trial populations were representative of the Medicare population on age, comorbidities and indications for cardiac pacing so generalizability is acceptable. Since historically the implantation and use of single chamber pacemakers have resulted in improved survival, these devices are generally considered the gold standard for patients with symptomatic bradycardia. Dual chamber pacemakers may be considered alternatives for individuals who may benefit from more complex pacing. This position is consistent with evidence based guidelines from ACC/AHA/HRS (2008) and ESC (2007) that state that permanent cardiac pacemakers for symptomatic bradycardia due to either SND or third degree and advanced second degree AVB are recommended.
Shared decision making between the physician and patient remains important for device choice on an individual basis. We expect that physicians will carefully document the need for dual chamber pacemakers in the medical record, and that patients will be informed of the potentially higher complication rates following implantation of dual chamber devices as reported in the randomized controlled trials. The use of evidence-based guidelines and expert consensus statements may also help to inform the device selection process.
Based on our review of the published evidence, we did not find specific evidence to support making many modifications to the list of specific non-covered indications identified in section 20.8 of the NCD Manual. Due to changes to the covered indications, however, we are revising the descriptions of the non-covered indications for clarity and to ensure there is internal consistency with covered indications. For instance, we are eliminating “without significant symptoms” as used to describe certain non-covered indications in existing policy because we use “symptomatic” to describe covered indications.
We are specifically non-covering permanent implantable single chamber or dual chamber cardiac pacemakers for patients with reversible causes of bradycardia (such as electrolyte abnormalities, medications or drugs, or hypothermia), since reversible causes of bradycardia are transient and not permanent in nature. We are non-covering permanent implantable single chamber or dual chamber cardiac pacemakers for patients with asymptomatic first degree AVB. Epstein and colleagues noted that “there is little evidence to suggest that pacemakers improve survival in patients with isolated first-degree AV block” and further noted: “Permanent pacemaker implantation is not indicated for asymptomatic first-degree AV block (Epstein, 2008).” Based on the public comments received, we are leaving the coverage determination under section 1862(a)(1)(A) to local Medicare contractors for prophylactic pacemaker use following recovery from acute myocardial infarction during which there was temporary complete (third-degree) and/or Mobitz Type II second-degree AVB in association with bundle branch block; and prolonged P-R intervals with atrial fibrillation (without third-degree atrioventricular block).
While pacemaker longevity has increased over the past few decades, replacement of a permanent cardiac pacemaker is occasionally needed due to wear or damage to one or more components (battery, generator, or leads). We did not find any specific evidence or recommendations on how to replace these devices or on which replacement devices should be used. Given the nature of the reasons for replacements, prudent clinical judgment should be used on an individual basis, along with documentation in the medical record on the initial indication for the pacemaker, the reason for replacement, and the justification of the type of replacement device selected. In rare instances, historical medical records may not be immediately available; however, adequate documentation should still be maintained to explain the existing circumstances and situation.
Disparities
Gender differences have been reported for various cardiac interventions (Jacobs, 2003) and implanted devices (Curtis, 2007). For cardiac pacemakers, Nowak and colleagues (2010) reported that “gender has an impact [on] pacemaker implantation, with less favourable outcomes for women.” CMS will monitor utilization of permanent implanted cardiac pacemakers for gender differences in the Medicare population. If gender differences are detected, then modifications may be considered.
Summary
There is an established and accepted association between single chamber cardiac pacemaker use, survival, and improvement in health outcomes. Historically, the use of single chamber pacemakers has been shown to improve survival for treatment of symptomatic bradycardia. Studies have demonstrated that health outcomes are similar for individuals that receive dual chamber pacemakers compared with single chamber pacemakers for treatment of symptomatic bradycardia. A dual chamber pacemaker may be considered as an alternative for individuals who may benefit from more complex pacing. There is some indication of better secondary outcomes from the use of dual chamber pacemakers, including reduction in occurrence of atrial fibrillation and improved quality of life. The choice of device may be influenced by reduced occurrence of AF and higher perioperative complications seen with dual chamber pacemakers, emphasizing the importance of informed, shared decision making between the physician and patient. Therefore, we have determined that permanent implantable single chamber and dual chamber cardiac pacemakers are reasonable and necessary subject to the conditions described in our conclusion below.
IX. Conclusion
The Centers for Medicare & Medicaid Services (CMS) has determined that the evidence is sufficient to conclude that implanted permanent cardiac pacemakers, single chamber or dual chamber, are reasonable and necessary for the treatment of non-reversible symptomatic bradycardia due to sinus node dysfunction and second and/or third degree atrioventricular block. Symptoms of bradycardia are symptoms that can be directly attributable to a heart rate less than 60 beats per minute (for example: syncope, seizures, congestive heart failure, dizziness, or confusion).
Therefore, the following indications are covered for implanted permanent single chamber or dual chamber cardiac pacemakers:
- Documented non-reversible symptomatic bradycardia due to sinus node dysfunction.
- Documented non-reversible symptomatic bradycardia due to second degree and/or third degree atrioventricular block.
The following indications are non-covered since there is insufficient evidence to conclude that implanted permanent cardiac pacemakers, single chamber or dual chamber, are reasonable and necessary:
- Reversible causes of bradycardia such as electrolyte abnormalities, medications or drugs, and hypothermia.
- Asymptomatic first degree atrioventricular block.
- Asymptomatic sinus bradycardia.
- Asymptomatic sino-atrial block or asymptomatic sinus arrest.
- Ineffective atrial contractions (e.g., chronic atrial fibrillation or flutter, or giant left atrium) without symptomatic bradycardia.
- Asymptomatic second degree atrioventricular block of Mobitz Type I unless the QRS complexes are prolonged or electrophysiological studies have demonstrated that the block is at or beyond the level of the His Bundle (a component of the electrical conduction system of the heart).
- Syncope of undetermined cause.
- Bradycardia during sleep.
- Right bundle branch block with left axis deviation (and other forms of fascicular or bundle branch block) without syncope or other symptoms of intermittent atrioventricular block.
- Asymptomatic bradycardia in post-myocardial infarction patients about to initiate long-term beta-blocker drug therapy.
- Frequent or persistent supraventricular tachycardias, except where the pacemaker is specifically for the control of the tachycardia.
- A clinical condition in which pacing takes place only intermittently and briefly, and which is not associated with a reasonable likelihood that pacing needs will become prolonged.
Medicare Administrative Contractors will determine coverage under section 1862(a)(1)(A) of the Social Security Act for any other indications for the implantation and use of single chamber or dual chamber cardiac pacemakers that are not specifically addressed in this national coverage determination.
Appendix A
General Methodological Principles of Study Design
When making national coverage determinations, CMS evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that an item or service falling within a benefit category is reasonable and necessary for the diagnosis or treatment of an illness or injury or to improve the functioning of a malformed body member. The overall objective for the critical appraisal of the evidence is to determine to what degree we are confident that: 1) the specific assessment questions can be answered conclusively; and 2) the intervention will improve health outcomes for patients.
We divide the assessment of clinical evidence into three stages: 1) the quality of the individual studies; 2) the generalizability of findings from individual studies to the Medicare population; and 3) overarching conclusions that can be drawn from the body of the evidence on the direction and magnitude of the intervention’s potential risks and benefits.
The methodological principles described below represent a broad discussion of the issues we consider when reviewing clinical evidence. However, it should be noted that each coverage determination has its unique methodological aspects.
Assessing Individual Studies
Methodologists have developed criteria to determine weaknesses and strengths of clinical research. Strength of evidence generally refers to: 1) the scientific validity underlying study findings regarding causal relationships between health care interventions and health outcomes; and 2) the reduction of bias. In general, some of the methodological attributes associated with stronger evidence include those listed below.
- Use randomization (allocation of patients to either intervention or control group) in order to minimize bias.
- Use contemporaneous control groups (rather than historical controls) in order to ensure comparability between the intervention and control groups.
- Use prospective (rather than retrospective) studies to ensure a more thorough and systematical assessment of factors related to outcomes.
- Use larger sample sizes in studies to help ensure adequate numbers of patients are enrolled to demonstrate both statistically significant as well as clinically significant outcomes that can be extrapolated to the Medicare population. Sample size should be large enough to make chance an unlikely explanation for what was found.
Use masking (blinding) to ensure patients and investigators do not know to which group patients were assigned (intervention or control). This is important especially in subjective outcomes, such as pain or quality of life, where enthusiasm and psychological factors may lead to an improved perceived outcome by either the patient or assessor.
Regardless of whether the design of a study is a randomized controlled trial, a non-randomized controlled trial, a cohort study or a case-control study, the primary criterion for methodological strength or quality is the extent to which differences between intervention and control groups can be attributed to the intervention studied. This is known as internal validity. Various types of bias can undermine internal validity. These include:
- Different characteristics between patients participating and those theoretically eligible for study but not participating (selection bias),
- Co-interventions or provision of care apart from the intervention under evaluation (performance bias),
- Differential assessment of outcome (detection bias), and
- Occurrence and reporting of patients who do not complete the study (attrition bias).
In principle, rankings of research design have been based on the ability of each study design category to minimize these biases. A randomized controlled trial minimizes systematic bias (in theory) by selecting a sample of participants from a particular population and allocating them randomly to the intervention and control groups. Thus, in general, randomized controlled studies have been typically assigned the greatest strength, followed by non-RCTs and controlled observational studies. The design, conduct and analysis of trials are important factors as well. For example, a well designed and conducted observational study with a large sample size may provide stronger evidence than a poorly designed and conducted randomized controlled trial with a small sample size. The following is a representative list of study designs (some of which have alternative names) ranked from most to least methodologically rigorous in their potential ability to minimize systematic bias.
- Randomized controlled trials
- Non-randomized controlled trials
- Prospective cohort studies
- Retrospective case control studies
- Cross-sectional studies
- Surveillance studies (e.g., using registries or surveys)
- Consecutive case series
- Single case reports
When there are merely associations but not causal relationships between a study’s variables and outcomes, it is important not to draw causal inferences. Confounding refers to independent variables that systematically vary with the causal variable. This distorts measurement of the outcome of interest because its effect size is mixed with the effects of other extraneous factors. For observational, and in some cases randomized controlled trials, the method in which confounding factors are handled (either through stratification or appropriate statistical modeling) are of particular concern. For example, in order to interpret and generalize conclusions to our population of Medicare patients, it may be necessary for studies to match or stratify their intervention and control groups by patient age or co-morbidities.
Methodological strength is, therefore, a multidimensional concept that relates to the design, implementation and analysis of a clinical study. In addition, thorough documentation of the conduct of the research, particularly study selection criteria, rate of attrition and process for data collection, is essential for CMS to adequately assess and consider the evidence.
Generalizability of Clinical Evidence to the Medicare Population
The applicability of the results of a study to other populations, settings, treatment regimens and outcomes assessed is known as external validity. Even well-designed and well-conducted trials may not supply the evidence needed if the results of a study are not applicable to the Medicare population. Evidence that provides accurate information about a population or setting not well represented in the Medicare program would be considered but would suffer from limited generalizability.
The extent to which the results of a trial are applicable to other circumstances is often a matter of judgment that depends on specific study characteristics, primarily the patient population studied (age, sex, severity of disease and presence of co-morbidities) and the care setting (primary to tertiary level of care, as well as the experience and specialization of the care provider). Additional relevant variables are treatment regimens (dosage, timing and route of administration), co-interventions or concomitant therapies, and type of outcome and length of follow-up.
The level of care and the experience of the providers in the study are other crucial elements in assessing a study’s external validity. Trial participants in an academic medical center may receive more or different attention than is typically available in non-tertiary settings. For example, an investigator’s lengthy and detailed explanations of the potential benefits of the intervention and/or the use of new equipment provided to the academic center by the study sponsor may raise doubts about the applicability of study findings to community practice. Given the evidence available in the research literature, some degree of generalization about an intervention’s potential benefits and harms is invariably required in making coverage determinations for the Medicare population. Conditions that assist us in making reasonable generalizations are biologic plausibility, similarities between the populations studied and Medicare patients (age, sex, ethnicity and clinical presentation) and similarities of the intervention studied to those that would be routinely available in community practice.
A study’s selected outcomes are an important consideration in generalizing available clinical evidence to Medicare coverage determinations. One of the goals of our determination process is to assess health outcomes. We are interested in the results of changed patient management not just altered management. These outcomes include resultant risks and benefits such as increased or decreased morbidity and mortality. In order to make this determination, it is often necessary to evaluate whether the strength of the evidence is adequate to draw conclusions about the direction and magnitude of each individual outcome relevant to the intervention under study. In addition, it is important that an intervention’s benefits are clinically significant and durable, rather than marginal or short-lived. Generally, an intervention is not reasonable and necessary if its risks outweigh its benefits.
If key health outcomes have not been studied or the direction of clinical effect is inconclusive, we may also evaluate the strength and adequacy of indirect evidence linking intermediate or surrogate outcomes to our outcomes of interest.
Assessing the Relative Magnitude of Risks and Benefits
Generally, an intervention is not reasonable and necessary if its risks outweigh its benefits. Health outcomes are one of several considerations in determining whether an item or service is reasonable and necessary. For most determinations, CMS evaluates whether reported benefits translate into improved health outcomes. CMS places greater emphasis on health outcomes actually experienced by patients, such as quality of life, functional status, duration of disability, morbidity and mortality, and less emphasis on outcomes that patients do not directly experience, such as intermediate outcomes, surrogate outcomes, and laboratory or radiographic responses. The direction, magnitude and consistency of the risks and benefits across studies are also important considerations. Based on the analysis of the strength of the evidence, CMS assesses the relative magnitude of an intervention or technology’s benefits and risk of harm to Medicare beneficiaries.
Appendix B
Table of Trials
| Author, Year |
Baseline |
Primary Outcome |
Secondary Outcomes |
Complications |
|---|
Connolly, 2000
Canadian Trial of Physiologic Pacing Investigators (CTOPP)
single ventricular pacing (n = 1474) vs physiologic pacing [dual or atrial (5.2%), n = 1094] |
mean age = 73 years; 59% men; sinoatrial node disease = 40%; AV block = 60% |
stroke or cv death; annual rate of stroke or cv death was 5.5% vs 4.9% respectively; RRR 9.5% 95% CI -10.5-25.7%; p = 0.33. mean follow-up=3 years. |
annual rate of all cause death 6.6% vs 6.3% respectively; RRR 0.9% 95%CI -18.1-16.8%; p=0.92. annual rate of atrial fib 6.6% vs 5.3% respectively; RRR 18% 95% CI 0.3-32.6%; p = 0.05. |
any perioperative 3.8% vs 9.0% respectively; p < 0.001. |
Lamas, 1998 Pacemaker Selection in the Elderly (PASE) single vent chamber (n = 204) vs dual chamber programming (n = 203) |
mean age=76 years; 60% men; sinus node 43%; AV block 49% |
health related QOL SF-36; “improved significantly after pacemaker implantation (P < 0.001), but there were no differences between the two pacing modes in either the quality of life…” |
death all causes 17% vs 16%; p = 0.95. stroke or death 19% vs 17%; p = 0.75. atrial fib 19% vs 17%; p = 0.80. |
complications not reported. |
Lamas, 2002 Mode Selection Trial in Sinus Node Dysfunction (MOST) dual chamber (n = 1014) vs programming (ventricular n = 996) |
median age=74 years; 52% men; sinus node dysfunction |
death any cause or nonfatal stroke; 21.5% vs 23%, respectively; HR 0.93 95% CI 0.78-1.13; p = 0.48. median follow-up = 33 months. |
all death 19.7% vs 20.5% respectively, HR 0.97 95% CI 0.8-1.18. atrial fibrillation 21.4% vs 27.1% respectively, HR 0.79; 95% CI 0.66-0.94; p = 0.008. |
perioperative 30d complication rate 4.8% (all patients received dual chamber). |
Nielsen, 2011 Danish Multicenter Randomized Trial on Single Lead Atrial Pacing versus Dual Chamber Pacing in Sick Sinus Syndrome (DANPACE) single lead atrial (n = 707) to dual chamber (n = 708) |
mean age = 73 years; 35% men; sick sinus syndrome |
death any cause; 29.6% vs 27.3% respectively; HR 1.06 95% CI 0.88-1.29; p = 0.53. mean follow-up = 5.4 years. |
paroxysmal atrial fib 28.4% vs 23% respectively, HR 1.27 95% CI 1.03-1.56; p = 0.024. chronic atrial fib 11.2% vs 10.7% respectively, HR 1.02 95% CI 0.74-1.39; p = 0.93. stroke and heart failure did not differ. |
complications not reported. |
Toff, 2005 United Kingdom Pacing and Cardiovascular Events (UKPACE) single ventricular (n = 1009) to dual chamber (n = 1012) |
mean age=80 years; 57% men; 2nd AV block 26.1%; complete AV block 73.3%; 83% symptomatic |
death all causes; mean annual mortality 7.2% vs 7.4%; respectively, HR 0.96 95% CI 0.83-1.11; p = 0.56. median follow-up = 4.6 years. |
no significant differences in atrial fib, heart failure, stroke/TIA/thromboembolism. annual rate atrial fib 3.0% vs 2.8% HR 1.05 95% CI 0.78-1.41). |
procedure complications single 3.5% vs dual 7.8%; p < 0.001. |
Appendix C
References Excluded from Analysis
Study Design, Sample size (n<50)
Attuel P, Pellerin D, Mugica J and Coumel P. DDD pacing: an effective treatment modality for recurrent atrial arrhythmias. Pacing Clin Electrophysiol. 1988; 11: 1647-54.
Dhingra RC, Denes P, Wu D, Chuquimia R and Rosen KM. The significance of second degree atrioventricular block and bundle branch block. Observations regarding site and type of block. Circulation. 1974; 49: 638-46.
Gammage M, Schofield S, Rankin I, Bennett M, Coles P and Pentecost B. Benefit of single setting rate responsive ventricular pacing compared with fixed rate demand pacing in elderly patients. Pacing Clin Electrophysiol. 1991; 14: 174-80.
Kay R, Estioko M and Wiener I. Primary sick sinus syndrome as an indication for chronic pacemaker therapy in young adults: incidence, clinical features, and long-term evaluation. Am Heart J. 1982; 103: 338-42.
Ouali S, Neffeti E, Ghoul K, et al. DDD versus VVIR pacing in patients, ages 70 and over, with complete heart block. Pacing Clin Electrophysiol. 2010; 33: 583-9.
Shohat-Zabarski R, Iakobishvili Z, Kusniec J, Mazur A and Strasberg B. Paroxysmal atrioventricular block: clinical experience with 20 patients. Int J Cardiol. 2004; 97: 399-405.
Steinbach M, Douchet MP, Bakouboula B, Bronner F and Chauvin M. Outcome of patients aged over 75 years who received a pacemaker to treat sinus node dysfunction. Arch Cardiovasc Dis. 2011; 104: 89-96.
Tachyarrhythmias, Atrial Fibrillation/Flutter, Resynchronization Therapy, Biventricular Pacing, Other Conditions
Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002; 346: 1845-53.
Albertsen AE, Nielsen JC, Poulsen SH, et al. Biventricular pacing preserves left ventricular performance in patients with high-grade atrio-ventricular block: a randomized comparison with DDD(R) pacing in 50 consecutive patients. Europace. 2008; 10: 314-320.
Barold SS, Wyndham CR, Kappenberger LL, Abinader EG, Griffin JC and Falkoff MD. Implanted atrial pacemakers for paroxysmal atrial flutter. Long-term efficacy. Ann Intern Med. 1987; 107: 144-9.
Elkayam LU, Koehler JL, Sheldon TJ, Glotzer TV, Rosenthal LS and Lamas GA. The Influence of Atrial and Ventricular Pacing on the Incidence of Atrial Fibrillation: A Meta-Analysis. Pacing Clin Electrophysiol. 2011.
Fananapazir L, Cannon RO, 3rd, Tripodi D and Panza JA. Impact of dual-chamber permanent pacing in patients with obstructive hypertrophic cardiomyopathy with symptoms refractory to verapamil and beta-adrenergic blocker therapy. Circulation. 1992; 85: 2149-61.
Fisher JD, Johnston DR, Furman S, Mercando AD and Kim SG. Long-term efficacy of antitachycardia pacing for supraventricular and ventricular tachycardias. Am J Cardiol. 1987; 60: 1311-6.
Galve E, Sambola A, Saldana G, et al. Late benefits of dual-chamber pacing in obstructive hypertrophic cardiomyopathy: a 10-year follow-up study. Heart. 2010; 96: 352-6.
Gillis AM, Koehler J, Morck M, Mehra R and Hettrick DA. High atrial antitachycardia pacing therapy efficacy is associated with a reduction in atrial tachyarrhythmia burden in a subset of patients with sinus node dysfunction and paroxysmal atrial fibrillation. Heart Rhythm. 2005; 2: 791-6.
Herre JM, Griffin JC, Nielsen AP, et al. Permanent triggered antitachycardia pacemakers in the management of recurrent sustained ventricular tachycardia. J Am Coll Cardiol. 1985; 6: 206-14.
Jeanrenaud X, Goy JJ and Kappenberger L. Effects of dual-chamber pacing in hypertrophic obstructive cardiomyopathy. Lancet. 1992; 339: 1318-23.
Kappenberger L, Linde C, Daubert C, et al. Pacing in hypertrophic obstructive cardiomyopathy. A randomized crossover study. PIC Study Group. Eur Heart J. 1997; 18: 1249-56.
Kass DA, Chen CH, Curry C, et al. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999; 99: 1567-73.
Leclercq C, Cazeau S, Lellouche D, et al. Upgrading from single chamber right ventricular to biventricular pacing in permanently paced patients with worsening heart failure: The RD-CHF Study. Pacing Clin Electrophysiol. 2007; 30 Suppl 1: S23-30.
Lee MA, Weachter R, Pollak S, et al. The effect of atrial pacing therapies on atrial tachyarrhythmia burden and frequency: results of a randomized trial in patients with bradycardia and atrial tachyarrhythmias. J Am Coll Cardiol. 2003; 41: 1926-32.
Maron BJ, Nishimura RA, McKenna WJ, Rakowski H, Josephson ME and Kieval RS. Assessment of permanent dual-chamber pacing as a treatment for drug-refractory symptomatic patients with obstructive hypertrophic cardiomyopathy. A randomized, double-blind, crossover study (M-PATHY). Circulation. 1999; 99: 2927-33.
Nishimura RA, Trusty JM, Hayes DL, et al. Dual-chamber pacing for hypertrophic cardiomyopathy: a randomized, double-blind, crossover trial. J Am Coll Cardiol. 1997; 29: 435-41.
Saad EB, Marrouche NF, Martin DO, et al. Frequency and associations of symptomatic deterioration after dual-chamber defibrillator implantation in patients with ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2002; 90: 79-82.
Steinberg JS, Fischer A, Wang P, et al. The clinical implications of cumulative right ventricular pacing in the multicenter automatic defibrillator trial II. J Cardiovasc Electrophysiol. 2005; 16: 359-65.
Sweeney MO, Hellkamp AS, Ellenbogen KA, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003; 107: 2932-7.
Thambo JB, Bordachar P, Garrigue S, et al. Detrimental ventricular remodeling in patients with congenital complete heart block and chronic right ventricular apical pacing. Circulation. 2004; 110: 3766-72.
Vanderheyden M, Goethals M, Anguera I, et al. Hemodynamic deterioration following radiofrequency ablation of the atrioventricular conduction system. Pacing Clin Electrophysiol. 1997; 20: 2422-8.
Wilkoff BL, Cook JR, Epstein AE, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002; 288: 3115-23.
Zeltser D, Justo D, Halkin A, et al. Drug-induced atrioventricular block: prognosis after discontinuation of the culprit drug. J Am Coll Cardiol. 2004; 44: 105-8.
Intermediate Outcomes/Other Outcomes
Albertsen AE, Nielsen JC, Poulsen SH, et al. DDD(R)-pacing, but not AAI(R)-pacing induces left ventricular desynchronization in patients with sick sinus syndrome: tissue-Doppler and 3D echocardiographic evaluation in a randomized controlled comparison. Europace. 2008; 10: 127-33.
Brecker SJ, Xiao HB, Sparrow J and Gibson DG. Effects of dual-chamber pacing with short atrioventricular delay in dilated cardiomyopathy. Lancet. 1992; 340: 1308-12.
Choo WK and Gupta S. The impact of NICE (UK) recommendations on outcomes of cardiac pacemaker implantations - a single-centre, district hospital experience. J Eval Clin Pract. 2011.
Dorenkamp M, Breitwieser C, Morguet AJ, Seegers J, Behrens S and Zabel M. T-wave alternans testing in pacemaker patients: comparison of pacing modes and long-term prognostic relevance. Pacing Clin Electrophysiol. 2011; 34: 1054-62.
Kafkas N, Patsilinakos S, Makris K, et al. Brain natriuretic peptide: a marker of cardiac dysfunction with ventricular or dual-chamber pacing. Acta Cardiol. 2011; 66: 589-94.
Knight BP, Gersh BJ, Carlson MD, et al. Role of permanent pacing to prevent atrial fibrillation: science advisory from the American Heart Association Council on Clinical Cardiology (Subcommittee on Electrocardiography and Arrhythmias) and the Quality of Care and Outcomes Research Interdisciplinary Working Group, in collaboration with the Heart Rhythm Society. Circulation. 2005; 111: 240-3.
Kristensen L, Nielsen JC, Mortensen PT, Pedersen AK and Andersen HR. Evaluation of pacemaker telemetry as a diagnostic feature for detecting atrial tachyarrhythmias in patients with sick sinus syndrome. Europace. 2004; 6: 580-5.
Lelakowski J, Majewski J, Malecka B, Bednarek J, Stypula P and Szeglowski M. Retrospective analysis of reasons for failure of DDD pacemaker implantation in patients operated on between 1993 and 2005. Cardiol J. 2007; 14: 155-9.
Linde-Edelstam C, Nordlander R, Pehrsson SK and Ryden L. A double-blind study of submaximal exercise tolerance and variation in paced rate in atrial synchronous compared to activity sensor modulated ventricular pacing. Pacing Clin Electrophysiol. 1992; 15: 905-15.
Maurer G, Torres MA, Corday E, Haendchen RV and Meerbaum S. Two-dimensional echocardiographic contrast assessment of pacing-induced mitral regurgitation: relation to altered regional left ventricular function. J Am Coll Cardiol. 1984; 3: 986-91.
Nahlawi M, Waligora M, Spies SM, Bonow RO, Kadish AH and Goldberger JJ. Left ventricular function during and after right ventricular pacing. J Am Coll Cardiol. 2004; 44: 1883-8.
Nery PB, Fernandes R, Nair GM, et al. Device-related infection among patients with pacemakers and implantable defibrillators: incidence, risk factors, and consequences. J Cardiovasc Electrophysiol. 2010; 21: 786-90.
Quirino G, Giammaria M, Corbucci G, et al. Diagnosis of paroxysmal atrial fibrillation in patients with implanted pacemakers: relationship to symptoms and other variables. Pacing Clin Electrophysiol. 2009; 32: 91-8.
Rubaj A, Rucinski P, Sodolski T, et al. Comparison of the acute hemodynamic effect of right ventricular apex, outflow tract, and dual-site right ventricular pacing. Ann Noninvasive Electrocardiol. 2010; 15: 353-9.
Sutton R and Bourgeois I. Cost benefit analysis of single and dual chamber pacing for sick sinus syndrome and atrioventricular block. An economic sensitivity analysis of the literature. Eur Heart J. 1996; 17: 574-82.
Search Time
Andersen HR, Nielsen JC, Thomsen PE, et al. Long-term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet. 1997; 350: 1210-6.
Kusumoto FM and Goldschlager N. Cardiac pacing. N Engl J Med. 1996; 334: 89-97.
Mymin D, Mathewson FA, Tate RB and Manfreda J. The natural history of primary first-degree atrioventricular heart block. N Engl J Med. 1986; 315: 1183-7.
Rasmussen K. Chronic sinus node disease: natural course and indications for pacing. Eur Heart J. 1981; 2: 455-9.
Strasberg B, Amat YLF, Dhingra RC, et al. Natural history of chronic second-degree atrioventricular nodal block. Circulation. 1981; 63: 1043-9.
Pacing Site, Device Programming
Carlson MD, Ip J, Messenger J, et al. A new pacemaker algorithm for the treatment of atrial fibrillation: results of the Atrial Dynamic Overdrive Pacing Trial (ADOPT). J Am Coll Cardiol. 2003; 42: 627-33.
Gillis AM, Purerfellner H, Israel CW, et al. Reducing unnecessary right ventricular pacing with the managed ventricular pacing mode in patients with sinus node disease and AV block. Pacing Clin Electrophysiol. 2006; 29: 697-705.
Heldman D, Mulvihill D, Nguyen H, et al. True incidence of pacemaker syndrome. Pacing Clin Electrophysiol. 1990; 13: 1742-50.
Hermida JS, Kubala M, Lescure FX, et al. Atrial septal pacing to prevent atrial fibrillation in patients with sinus node dysfunction: results of a randomized controlled study. Am Heart J. 2004; 148: 312-7.
Israel CW, Hugl B, Unterberg C, et al. Pace-termination and pacing for prevention of atrial tachyarrhythmias: results from a multicenter study with an implantable device for atrial therapy. J Cardiovasc Electrophysiol. 2001; 12: 1121-8.
Lamas GA, Knight JD, Sweeney MO, et al. Impact of rate-modulated pacing on quality of life and exercise capacity evidence from the Advanced Elements of Pacing Randomized Controlled Trial (ADEPT). Heart Rhythm. 2007; 4: 1125-32.
Madan N and Saksena S. Long-term rhythm control of drug-refractory atrial fibrillation with "hybrid therapy" incorporating dual-site right atrial pacing, antiarrhythmic drugs, and right atrial ablation. Am J Cardiol. 2004; 93: 569-75.
Nguyen CT, Moreno-Cabral CE and Mahnke CB. Pacemaker upgrade causing new-onset heart failure in a patient with complete congenital atrioventricular block. Pediatr Cardiol. 2010; 31: 106-7.
Nielsen JC, Pedersen AK, Mortensen PT and Andersen HR. Programming a fixed long atrioventricular delay is not effective in preventing ventricular pacing in patients with sick sinus syndrome. Europace. 1999; 1: 113-20.
Nikoo MH, Ghaedian MM, Kafi M, et al. Effects of right ventricular septal versus apical pacing on plasma natriuretic peptide levels. J Cardiovasc Dis Res. 2011; 2: 104-9.
Nitardy A, Langreck H, Dietz R and Stockburger M. Reduction of right ventricular pacing in patients with sinus node dysfunction through programming a long atrioventricular delay along with the DDIR mode. Clin Res Cardiol. 2009; 98: 25-32.
Olshansky B, Day JD, Moore S, et al. Is dual-chamber programming inferior to single-chamber programming in an implantable cardioverter-defibrillator? Results of the INTRINSIC RV (Inhibition of Unnecessary RV Pacing With AVSH in ICDs) study. Circulation. 2007; 115: 9-16.
Saksena S, Prakash A, Hill M, et al. Prevention of recurrent atrial fibrillation with chronic dual-site right atrial pacing. J Am Coll Cardiol. 1996; 28: 687-94.
Sanfins V, Alves A, Rodrigues B, et al. RIVER: Portuguese registry to monitor unnecessary right ventricular pacing. Rev Port Cardiol. 2010; 29: 581-9.
Sharma AD, Rizo-Patron C, Hallstrom AP, et al. Percent right ventricular pacing predicts outcomes in the DAVID trial. Heart Rhythm. 2005; 2: 830-4.
Sulke N, Chambers J, Dritsas A and Sowton E. A randomized double-blind crossover comparison of four rate-responsive pacing modes. J Am Coll Cardiol. 1991; 17: 696-706.
Sweeney MO, Bank AJ, Nsah E, et al. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007; 357: 1000-8.
Sweeney MO, Ellenbogen KA, Miller EH, Sherfesee L, Sheldon T and Whellan D. The Managed Ventricular pacing versus VVI 40 Pacing (MVP) Trial: clinical background, rationale, design, and implementation. J Cardiovasc Electrophysiol. 2006; 17: 1295-8.
Sweeney MO, Hellkamp AS, Ellenbogen KA, et al. Prospective randomized study of mode switching in a clinical trial of pacemaker therapy for sinus node dysfunction. J Cardiovasc Electrophysiol. 2004; 15: 153-60.
Tops LF, Schalij MJ and Bax JJ. The effects of right ventricular apical pacing on ventricular function and dyssynchrony implications for therapy. J Am Coll Cardiol. 2009; 54: 764-76.
Veasey RA, Arya A, Silberbauer J, Sharma V, Lloyd GW, Patel NR, Sulke AN. The relationship between right ventricular pacing and atrial fibrillation burden and disease progression in patients with paroxysmal atrial fibrillation: the long-MinVPACE study. Europace. 2011 Jun;13(6):815-20. doi: 10.1093/europace/euq463. Epub 2011 Jan 5.
Editorials, Commentaries
Connelly DT and Steinhaus DM. Mobitz type I atrioventricular block: an indication for permanent pacing? Pacing Clin Electrophysiol. 1996; 19: 261-4.
Das MK, Dandamudi G and Steiner HA. Modern pacemakers: hope or hype? Pacing Clin Electrophysiol. 2009; 32: 1207-21.
El Gamal M. Atrial pacing, the forgotten pacing mode. Neth Heart J. 2008; 16: S25-7. Abstract
Modi S, Krahn A and Yee R. Current concepts in pacing 2010-2011: the right and wrong way to pace. Curr Treat Options Cardiovasc Med. 2011; 13: 370-84.
Appendix D
ECG Grid: Waves, Intervals, and Segments
Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Walker HK, Hall WD, Hurst JW, editors. Boston: Butterworths; 1990. Copyright © 1990, Butterworth Publishers, a division of Reed Publishing. NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
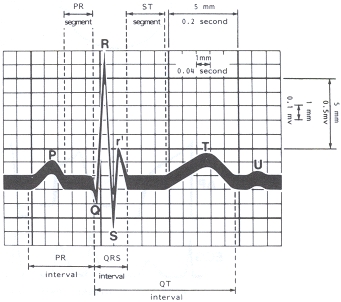
Figure 33.3
ECG grid. Waves, intervals, segments.
From: Chapter 33, Electrocardiography
Appendix E
NASPE/BPEG Generic Code for Antibradycardia, Adaptive-Rate, and Multisite Pacing
British Pacing and Electrophysiology Group. Recommendations for pacemaker prescription for symptomatic bradycardia. Report of a working party of the British Pacing and Electrophysiology Group. Br Heart J. 1991 Aug;66(2):185-91.
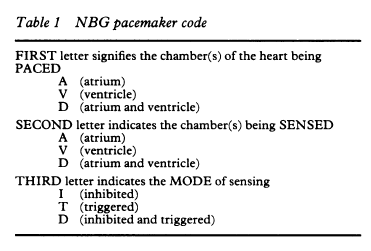
Bernstein AD, Daubert JC, Fletcher RD, Hayes DL, Lüderitz B, Reynolds DW, Schoenfeld MH, Sutton R. NASPE Position Statement: The Revised NASPE/BPEG Generic Code for Antibradycardia, Adaptive-Rate, and Multisite Pacing. J of Pacing and Clinical Electrophysiology. Vol. 25, No. 2, February 2002.
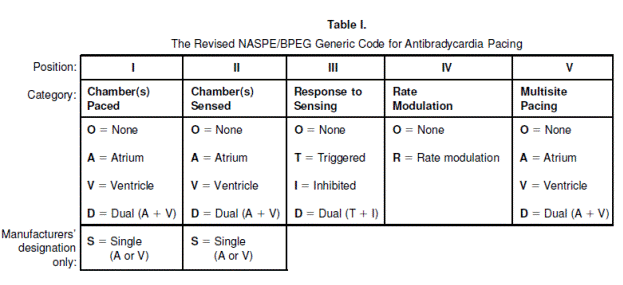
Appendix F
HRS/ACCF Expert Consensus Statement Classification of Recommendations
Reproduced from:
Gillis AM, Russo AM, Ellenbogen KA, Swerdlow CD, Olshansky B, Al-Khatib SM, Beshai JF, McComb JM, Nielsen JC, Philpott JM, Shen WK; Heart Rhythm Society; American College of Cardiology Foundation. HRS/ACCF expert consensus statement on pacemaker device and mode selection. Developed in partnership between the Heart Rhythm Society (HRS) and the American College of Cardiology Foundation (ACCF) and in collaboration with the Society of Thoracic Surgeons. Heart Rhythm. 2012 Aug;9(8):1344-65. doi: 10.1016/j.hrthm.2012.06.026.