To: CAG File #00074N
From: Sean Tunis, MD, MSc
Director, Coverage and Analysis Group
Jeffrey Shuren, MD, JD
Director, Division of Items and Devices
Sharon Hippler
Health Insurance Specialist, Coverage and Analysis Group
Subject: Coverage Decision Memorandum for Percutaneous Image Guided Breast Biopsy for Palpable Lesions
Date: April 12, 2002
This decision memo addresses a request for a national coverage decision received from Ethicon Endo-Surgery, Inc. The service for which coverage is requested is percutaneous image-guided breast biopsy for palpable lesions.
This memo serves five purposes: (1) outlines the epidemiology of breast cancer; (2) briefly describes the available methods of diagnosing breast cancer; (3) reviews the history of Medicare's coverage policies regarding percutaneous image-guided breast biopsy; (4) presents and analyzes the relevant scientific literature relating to the various methods of performing breast biopsies for palpable lesions; and (5) explains the rationale to issue a positive national coverage determination for lesions that are difficult to biopsy using palpation.
Clinical Background
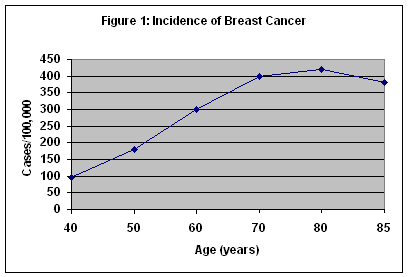
Breast cancer is the most common form of cancer in women in this country, and the second leading cause of cancer death. It is the most common cause of cancer deaths in women over 65 years of age. The incidence of breast cancer increases up to 80 years of age and plateaus between 80 and 85 years. (Figure 1) Although breast cancer is often perceived as a disease of middle-aged women, more than 50 percent of breast cancers occur in women 65 years of age and older. Because of increased comorbidities, the death rate for breast cancer in women over 65 years of age is nearly 50 percent. For all ages, over 40,000 women die from breast cancer each year. It is estimated that an American woman's lifetime risk of developing breast cancer is about one in eight.
Detection of Breast Cancer
A. Screening
Historically, most breast cancers present as an asymptomatic palpable breast lump. As the impact of breast cancer draws greater attention and treatment methods develop, the importance of early detection becomes apparent. Early detection relies on screening protocols involving physical examination of the breast by both the patient and the clinician, as well as imaging studies. Imaging primarily implies screening mammography although screening ultrasound is employed in some situations.1 The American Cancer Society and the National Cancer Institute currently recommend that women over 40 years of age undergo screening mammograms on an annual basis. Slightly fewer than half of the 90 million women over forty years of age undergo such screening. Currently, the increased practice of screening mammography has made an impact such that more than half of newly diagnosed breast cancers are detected when they are smaller than 2 cm. It is estimated that 5 cancers are detected for every 1000 women screened.
The American College of Radiology (ACR) employs a system for reporting mammography results. This Breast Imaging Reporting and Data System (BIRADS) provides a standardized lexicon with which radiologists may report their interpretation of a mammogram (Table 1). The radiologist may also convey information regarding the composition of the breast tissue by means of another BIRADS classification scheme (Table 2).
Table 1: BIRADS Grading of Mammograms
- Grade I: Negative
- Grade II: Benign finding
- Grade III: Probably benign
- Grade IV: Suspicious abnormality
- Grade V: Highly suggestive of malignant neoplasm
Table 2: BIRADS Classification of Breast Tissue Composition
- Class I: Fatty tissue
- Class II: Scattered fibroglandular densities
- Class III: Heterogeneously dense breast tissue
- Class IV: Extremely dense breast tissue
B. Diagnosis by Breast Biopsy
A radiographic abnormality may raise the suspicion of breast cancer but it does not provide a definitive diagnosis; confirmation by histopathology of the breast tissue is always required. Depending on the level of suspicion of the radiographic abnormality, the physician may advise the patient to follow the lesion radiographically, or to undergo a breast biopsy to obtain a specimen to be sent for histologic diagnosis. There are approximately one million breast biopsies performed in the United States each year. About 50% of these biopsies are performed to evaluate palpable lesions. For most of the 20th century, dominant, solid, palpable lesions were traditionally referred for surgical excision. Surgery, however, can be invasive, expensive, and often causes scarring and deformity of the breast. In light of the fact that the pathology report proves that 80% of palpable breast masses undergoing surgery are benign, the surgical excision route may become disadvantageous.
For cancerous lesions, the breakdown of histologic diagnoses is as follows: 80% ductal carcinoma, 10% lobular carcinoma, 5% medullary carcinoma, and 5% other histology. The most common histologic diagnoses are listed in Table 3.
Table 3: Histologic Diagnoses of Biopsied Breast Tissue
- Normal Breast Tissue Fibrocystic Change: A common finding involving morphologic changes which usually have no clinical significance
- Fibroadenoma: The most common benign breast tumor
- Atypical Ductal Hyperplasia (ADH): A noncancerous abnormality which causes an increased risk of developing carcinoma
- Lobular Carcinoma In Situ (LCIS): A proliferative breast lesion arising in the terminal ducts or ductules which causes a greatly increased risk of developing infiltrating carcinomas
- Ductal Carcinoma In Situ (DCIS): A proliferative breast lesion arising in the ducts which causes a greatly increased risk of developing infiltrating carcinomas
- Infiltrating Ductal Carcinoma (IDC): Accounts for about 80% of invasive breast cancers
- Infiltrating Lobular Carcinoma: Accounts for about 10% of invasive breast cancers
- Medullary Carcinoma: Accounts for about 5% of invasive breast cancers
C. Methods of Breast Biopsy
There are three methods of breast biopsy: (1) open surgical biopsy; (2) percutaneous2 palpation-guided breast biopsy; and (3) percutaneous image-guided breast biopsy.
Currently, the majority of breast biopsies performed are open surgical biopsies. Open surgical biopsies (OSB) are performed in an operating theater, usually following needle wire localization placed in the radiology suite. Open surgical biopsy has historically been accepted as the gold standard method of obtaining a specimen of breast tissue for histologic diagnosis. Typically, 5-25 gm of tissue is removed through a surgical incision site 3-6 cm in length. This large amount of tissue allows accuracy of the diagnosis, and at times can even be therapeutic.
OSB, however, is an imperfect gold standard, with an error rate estimated between 0.2 to as high as 20%3. Sources of error include incision error, localization error (e.g. dependent upon use of a wire), and tissue selection error. Other drawbacks include the fact that the technique can be disfiguring and time consuming.
Less invasive biopsy methods, however, are gradually increasing in use, often replacing surgical biopsy as the next diagnostic step for some radiographically detected lesions. Several of these methods employ palpation guidance, but some palpation-guided methods have been reported to have a lower accuracy than surgical biopsy. For example, palpation-guided fine needle aspiration (FNA) of breast masses has been reported to have a false negative rate of 0-35%, a false positive rate of 0-2%, and an insufficiency rate of 0-28%. In addition, FNA diagnoses are based on cytology - as a result, ductal carcinoma in situ and infiltrating ductal cancer cannot be distinguished by FNA. Diagnostic yield can be improved and false-positive biopsies can be reduced or eliminated with the use of core needle biopsy, as opposed to fine-needle aspiration. However, palpation-guided core needle biopsy has a false-negative rate of 0-36% and an insufficiency rate of 2-10%.
Image guidance systems provide a less invasive alternative to surgical biopsy and may be more accurate than biopsy methods employing guidance by palpation alone for some breast lesions. The problems of palpation-guided breast biopsy may in part reflect the difficulty of positioning the needle within some lesions. Image-guided biopsy addresses this problem by providing the clinician with a visualization of breast tissue areas that produced an abnormality on the initial mammogram. Image guidance may be either ultrasound or stereotactic in nature, and both may provide more accurate methods of positioning the needle within certain lesions than methods utilizing palpation guidance alone.
D. Percutaneous Image-Guided Breast Biopsy
There are several methods of percutaneous image-guided breast biopsy, including the following: (1) Directional, Vacuum Assisted Biopsy Performed with Imaging Guidance (DVAB); (2) Automated Surgical Biopsy; (3) Needle Core Biopsy; and (4) Stereotactic Large Core Excisional Biopsy.
1. Directional, Vacuum Assisted Biopsy Performed with Imaging Guidance (DVAB)
Image guided, directional vacuum assisted systems offer a method of obtaining breast tissue for histologic analysis by less invasive means than with open surgical excision. The patient typically lies prone on a table equipped with an imaging system with her breast protruding through a hole in the table.4 After administration of local anesthesia, the physician (radiologist or surgeon) makes a 3-5mm skin incision through which the probe is inserted. Employing image guidance (either stereotactic mammography or ultrasound), the probe is advanced to the tissue of mammographic concern. The vacuum draws tissue samples into the probe which are then cut, collected, and sent for pathology analysis. The amount of tissue removed is approximately 100 mg per core. Numerous tissue samples may be collected during the procedure. If an 11-gauge device is used, the clinician has the option of placing a radiopaque metal clip through the probe, to assist with future identification of the biopsy site. For biopsies performed to investigate microcalcifications, the tissue sample is radiographed to ensure presence of calcifications in the specimen.
2. Automated Surgical Biopsy
The patient lies prone on a stereotactic table and the breast is compressed. The device is deployed from the site offering the shortest distance from skin to lesion. After injection of local anesthetic agents, a 1-3 cm incision is made in the breast through which an open-ended cannula (10, 15, or 20 mm in diameter) containing a motorized oscillating blade is inserted. The cannula is advanced into the breast under stereotactic mammographic guidance (ultrasound guidance is not compatible with this technique). A cylinder of breast tissue is cut by an oscillating blade and then a wire loop cautery. The device is then removed from the breast and the detached plug of tissue is then extracted from the cannula. The tissue removed is a core of tissue from the skin to the abnormality. Radiographic examination of the specimen is then performed. If necessary, additional tissues are removed via the tract left at extraction of the cannula. If the entire mammographic abnormality is to be removed, the patient is repositioned supinely and the skin incision is then closed with sutures. One tissue sample is obtained during a procedure employing the cannula. These procedures are usually performed with local anesthesia, however, occasionally conscious sedation can be employed for extremely anxious patients.
3. Needle Core Biopsy with Image Guidance
Core needle biopsy involves deployment of a large core needle into the mammographically suspicious breast tissue by means of an automated gun. Image guidance can be either stereotactic or ultrasound. (Ultrasound is the predominant technique.) The patient lies prone on a table equipped with an imaging system with her breast protruding through an aperture in the table.5 After administration of local anesthesia, the physician (radiologist or surgeon) makes a small skin incision. A hollow needle is then inserted through this incision and directed towards the lesion employing imaging-guidance. When the needle is deemed to be in the proper position, the automated gun is fired to obtain a tissue sample. Numerous tissue samples (typically 10-20) may be obtained during a procedure. Total tissue removed is typically 10-15 mg per core. This technique is physician-dependent. For biopsies performed to investigate microcalcifications, the tissue sample is radiographed to ensure presence of calcifications in the specimen.
4. Stereotactic Large Core Excisional Biopsy
The device offers a method for performing wire localization and stereotactic excision of an intact, contiguous specimen of breast tissue up to 22 mm in diameter with minimal disruption of adjacent tissue or need for anesthesia (enhancing cosmesis and recovery). The patient lies prone on a stereotactic table, and the breast abnormality is targeted through the window of the compression plate using stereotactic mammographic guidance. After administering local anesthesia, the surgeon advances the localization needle into the suspicious lesion and deploys the localization wire. The surgeon then extends the incision to approximately 2 to 3 centimeters to accommodate the cutting cannula. To achieve clear margins, the diameter of the mammographic abnormality should be 3-4 mm less than the diameter of the coring cannula. The surgeon then advances the bladed stylet cannula portion of the device, spreading and separating the non-targeted tissue until it reaches the proximal aspect of the breast abnormality. The surgeon advances the cutting cannula and initiates specimen coring of the breast abnormality. This minimizes the disruption or removal of adjacent breast tissue between the skin and the abnormality. The surgeon advances the coring cannula manually past the localization wire and deploys the garrote wire to transect the distal aspect of the specimen. A non-fragmented specimen for pathological evaluation is captured within the cannula.
History of Medicare Coverage Policies
Coverage of percutaneous image-guided breast biopsy has long been determined at the local level by Medicare contractors. On December 7, 1999, CMS issued a national coverage decision memorandum for percutaneous image-guided breast biopsy, which can be found at www.cms.hhs.gov/coverage/8b3-h.asp. The decision provided coverage for percutaneous image-guidance breast biopsy using stereotactic or ultrasound systems for lesions that are (1) nonpalpable and (2) BIRADS III, IV, or V. Image-guidance systems included directional, vacuum assisted breast biopsy, automated surgical biopsy, and core needle biopsy. For palpable lesions, discretion regarding coverage was left to individual carriers.
Timeline of Recent CMS Activities
October 12, 2000, Ethicon Endo-Surgery, Inc. submitted a formal request to expand coverage of percutaneous image-guided breast biopsy to include palpable lesions.
January 11, 2001, CMS received an official position statement from the American College of Radiology. Received a position statement from Tristan Associates stating that palpability is irrelevant in using image-guidance.
January 30, 2001, CMS received an official position statement from the American Society of Breast Surgeons.
February 1, 2001, CMS received an official position statement from the American College of Surgery.
March 27, 2001, Met with Ethicon Endo-Surgery. Due date extended to May 14, 2001. Due dates subsequently extended to permit adequate time to review the evidence.
Summary of Evidence
The scientific literature was searched through Medline using combinations of the following terms: "image-guided breast biopsy," "nonpalpable lesions," "stereotactic," "breast cancer," and "breast lesion." A total of 80 articles were obtained. After excluding review articles, editorial, letters, and articles that did not constitute a trial or did not specify palpable lesions, a total of four articles were reviewed.
Pijnappel, et al. (1997) assessed the accuracy of image-guided large core needle biopsy in evaluating palpable and nonpalpable breast lesions in comparison with surgical excisional biopsy.6 The study population reported results from 27 palpable breast lesions and 76 nonpalpable lesions. Image guidance was either stereotactic or ultrasound in nature with 61% of palpable lesions and 96% of nonpalpable lesions undergoing stereotactic-guided biopsy. All 97 patients underwent subsequent surgery so that the histopathologic findings of the core biopsies could be correlated with the surgical specimens. The study demonstrated that for palpable lesions with inconclusive fine needle aspiration cytology, image guided core needle biopsy can accurately provide a diagnosis. For palpable lesions, the study demonstrated 100% agreement between the histopathological findings from core needle biopsy, using either ultrasound or stereotactic guidance, with surgical excision. In 102 (99%) of the 103 breast lesions, a correct choice for additional diagnostic procedure or definitive treatment could have been made upon histopathologic findings of core biopsy. In addition, all core biopsies of palpable lesions produced sufficient tissue for histological analysis in contrast to previously performed fine needle aspiration cytology. No information regarding statistical analysis was provided.
Hatada, et al. (1996) performed a retrospective study in order to determine whether ultrasound guidance improves the sensitivity and specificity of fine needle aspiration biopsy (FNAB) for patients with palpable breast masses.7 The study population included 107 patients (114 lesions) with palpable breast lesions that underwent ultrasound-guided FNAB and 138 patients (143 lesions) with palpable breast lesions that underwent palpation-guided FNAB (standard FNAB) at a university hospital. The diagnostic outcomes obtained by FNAB were then compared with the surgical findings and the diagnostic value of ultrasound-guided FNAB was evaluated. Using the Fornage method to evaluate all techniques, the sensitivity of ultrasound-guided FNAB was 89.3% (p < .001), the specificity was 82.9% (p < .001), and the accuracy was 86.9% (p < .001). In contrast, standard FNAB had 61.1% (p < .001) sensitivity, 73.3% (p < .001) specificity, and 65.0% (p < .001) accuracy rate. The inadequate biopsy rate for ultrasound-guided FNAB was 6.1% (p < .001) while standard FNAB had a 19.6% rate. Hatada, et al. also reported the findings on palpable breast lesions 2 cm or less in diameter and on palpable breast lesions larger than 2 cm. The authors reported that there was no significant difference of diagnostic accuracy for lesions larger than 2 cm between the ultrasound-guided FNAB and palpation-guided FNAB. For lesions 2 cm or less, the sensitivity and accuracy of ultrasound-guided FNAB were significantly higher than those of standard FNAB. Hatada, et al. concluded from their findings that ultrasound-guided FNAB may improve the preoperative diagnosis of small breast cancers, especially in patients with tumors that are 2 cm or less in diameter. The study, however, has several limitations: (1) it is a retrospective case series; (2) the subjects who received palpation-guided FNAB were biopsied earlier in time (1987 to 1991) than those subjects biopsied using image-guided FNAB (1992 to 1995); (3) there is no description of whether or not the indvidual(s) who compared cytopathologic diagnosis with surgical findings was masked; and (4) FNAB was used, which is not a biopsy technology commonly employed with image guidance in the United States today and which has a lower accuracy than other technologies.
Liberman, et al. (2000) attempted to determine the accuracy of percutaneous image-guided core biopsy in providing a decision for definitive treatment in women with palpable breast lesions.8 The study reported findings on 107 women (115 palpable lesions) who either underwent surgical excision following percutaneous image-guided core biopsy or were followed for a 2 year minimum. Age of patients ranged from 19-88 years, with a median of 47 years. BIRADS was category 4 in 55%, category 5 in 45%. Image guidance was performed with either sonographic or stereotactic methods. Palpable lesions were referred for image-guided biopsy at the discretion of the referring physician. For percutaneous image-guided malignant diagnoses, Liberman, et al. demonstrated a 97% agreement with surgical excision. For benign percutaneous image-guided diagnoses, 77% of the women demonstrated stability at follow-up. In addition, percutaneous image-guided core biopsy obviated additional diagnostic tissue sampling in 74% of the women enrolled in the study.9 Liberman, et al. concluded that percutaneous image-guided biopsy is useful for palpable lesions similar to those included in this study - specifically, palpable lesions that are small, deep, mobile or vaguely palpable and therefore, difficult to reliably place the needle in the lesion under guidance of palpation alone.
Doyle, et al. (1998) used a nonrandomized prospective study designed to assess the diagnostic accuracy of stereotactic-guided and ultrasound-guided large core needle biopsy (LCNB).10 During the time period of September 1994 to January 1997, 213 LCNB were performed on 204 women; 59 (28%) of the 213 LCNB were performed on palpable lesions. The authors reported that 31 (53%) of the 59 palpable lesions had positive LCNB. The absolute sensitivity and specificity along with the complete sensitivity and specificity were calculated. Doyle explained the absolute sensitivity and specificity to refer to the proportion of cancers proven at excision in which a definite diagnosis of cancer was reported on the core biopsy. Complete sensitivity and specificity referred to the number of proven cancers in which the core biopsy was also positive. The absolute sensitivity based on the excised cancers was 97% while the complete sensitivity was 100%. A 100% absolute specificity and 98.5% complete specificity were reported. The authors did not distinguish calculations for palpable lesions nor did they report any statistical analysis.
Position Statements
American College of Radiology
In response to the posting on the website, the American College of Radiology (ACR) submitted on January 11, 2001 a position statement on percutaneous image-guided breast biopsy for palpable lesions. The ACR stated that:
"...image guided breast biopsy...[should] be reimbursed by HCFA carriers, regardless of whether or not the patient has a history of a 'palpable' breast lesion."
The ACR explained that representatives have testified and commented that breast biopsy codes should be based on three factors, of which, none referred to palpability of a lesion as a criterion.11
In addition, a memo dated November 25, 1999 stated that:
"...the ACR feels strongly that it is in the best interest of Medicare beneficiaries, as well as all patients, that the image guided breast biopsy codes...be applicable both for non-palpable and palpable lesions."
American Society of Breast Surgeons
The American Society of Breast Surgeons also submitted an official position statement regarding percutaneous image-guided breast biopsy for palpable lesions:
"Image-guidance is an extremely useful adjunct in the performance of percutaneous biopsy of palpable breast lesions. Image-guidance confirms the proper placement of the biopsy device into the lesion (when core needle device is used), or immediately below the lesion (when vacuum-assisted device is used). Performing percutaneous breast biopsy procedures without the use of image-guidance may lead to false negative results since the biopsy device cannot be confirmed to be in the proper position to obtain tissue from the suspect mass. In most, if not all instances of image-guided biopsy of palpable lesions, ultrasound is the preferred image-guidance modality."
American College of Surgeons
In a February 13, 2001 letter to the agency, Dr. Henry Desmarais, Director of Health Policy and Advocacy of ACS wrote:
"..the College does not believe that image-guided breast biopsy should be considered the routine or typical way to approach palpable lesions. Instead, more traditional approaches can be used ( e.g. core needle biopsy). However, the College also believes it would be appropriate for Medicare to provide coverage for image-guided breast biopsies for some palpable lesions, those in which the yield and accuracy of the biopsy procedure would, in the opinion of the biopsying physician, be enhanced by doing the procedure with image guidance. For example, a patient may present with a relatively large palpable mass but the mammogram demonstrates that only a small portion of this mass contains clustered microcalcifications. Under such a circumstance, a non-guided biopsy might well yield a false negative result."
CMS Analysis
National coverage determinations (NCDs) are determinations by the Secretary with respect to whether or not a particular item or service is covered nationally under title XVIII of the Social Security Act. §1869(f)(1)(B). In order to be covered by Medicare, an item or service must fall within one or more benefit categories contained within Part A or Part B, and must not be otherwise excluded from coverage. Moreover, with limited exceptions, the expenses incurred for items or services must be "reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member." §1862(a)(1)(A).
We have fully examined the available scientific and clinical evidence submitted with the request for a national coverage decision. We have determined that the evidence is adequate to conclude that percutaneous image-guided breast biopsy is clinically effective, and, therefore, is reasonable and necessary, for diagnosing the histopathology and improving the management of patients with palpable lesions that are difficult to biopsy using palpation. The clinical studies suggest that such lesions may include those that are vaguely palpable, mobile, deep, or small, particularly less than 2 cm. Palpable lesions that demonstrate a small area of clustered microcalcifications on a mammogram may be difficult to biopsy using palpation alone and thus may warrant image-guided biopsy. Lesions that are difficult to biopsy using palpation are generally those that border on being nonpalpable; nonpalpable lesions are not amenable to palpation-guided biopsy. As discussed in our December 7, 1999 decision memorandum on nonpalpable lesions (www.cms.hhs.gov/coverage/8b3-h.asp), the diagnostic accuracy of percutaneous image-guided breast biopsy is similar to open surgical biopsy, the gold standard. Image guidance increases the diagnostic accuracy of the biopsy over palpation guidance in patients with palpable lesions that are difficult to biopsy using palpation. Although several specialty societies whose members use the technology support the use of image guidance to biopsy any palpable breast lesion, the evidence is not adequate to conclude that percutaneous image-guided breast biopsy increases diagnostic accuracy compared to palpation-guided breast biopsy for palpable lesions other than those that are difficult to biopsy using palpation.12 In making this determination we considered the following issues:
- Is the evidence adequate to determine whether percutaneous image-guided breast biopsy provides an accurate diagnosis for palpable breast masses?
- Is the evidence adequate to determine that for palpable breast masses, image guidance improves the diagnostic accuracy of biopsying compared to palpation alone (non-image guided)?
- For what patient population does the technique improve diagnostic accuracy?
- How would the diagnostic information affect patient management?
CMS considers several generally accepted methodological principles when assessing clinical studies of diagnostic tests. For example, we evaluate whether or not general methods of study design have been followed, such as calculating sample size a priori, specifying inclusion and exclusion criteria, describing the process for the selection of study participants and the ways in which the consistency of this process was maintained, comparing the diagnostic test to the appropriate gold standard, describing baseline characteristics of the participants, randomizing study subjects, masking of patients, investigators, and readers to the extent feasible, and performing appropriate statistical analyses.
When evaluating a diagnostic test, an important consideration is an assessment of the accuracy and technical characteristics of the test as compared to other diagnostic modalities. The optimal comparison is between the test under review and a gold standard, if one exists. The measures relied upon to determine accuracy include sensitivity (probability of a positive test result in a patient with a disease) and specificity (the probability of a negative test result in a patient who does not have the disease). An increase in sensitivity does not necessarily mean that a test is more accurate. Specificity must also be evaluated when determining if one test is more accurate than the other, because a highly specific test minimizes the number of false positive.13 In addition, increasing sensitivity or specificity is often accomplished at the expense of the other. However, even though a diagnostic test may be very accurate, if the information provided by the test is not useful in the management of the patients' specific medical problem, CMS may determine that the test is not reasonable and necessary. 42 C.F.R. §410.32.
A reliable study design for evaluating percutaneous image-guided breast biopsy would evaluate two populations of similar patients with palpable breast lesions who are randomly assigned to either: (1) percutaneous breast biopsy with no image guidance or (2) percutaneous breast biopsy with image-guidance. Both groups would also undergo open surgical biopsy, and the resulting sensitivity and specificity would be evaluated. This type of study has not been conducted. The lack of this type of study results in some uncertainty regarding the procedure's effectiveness because of potential biases in study design.
Because there are different methods of image-guidance (stereotaxis, ultrasound, etc), as well as different types of percutaneous biopsy, data on one method may not be generalizable to other methods. In general, it would be ideal to see data for each type of image guidance for each biopsy method, or at least a compelling rationale of why data may be generalizable. Such information does not exist. Table 4 summarizes the information presently available.
Table 4: Study Summary
| STUDY |
BIOPSY METHOD |
NUMBER OF LESIONS |
CALCULATIONS |
|---|
Hatada
(case series) |
US-guided FNAB with 22-Gauge needle Standard (non-image Guided) FNAB with 22-Gauge needle |
US-guided FNAB:
114 palpable lesions Standard FNAB:
143 palpable lesions |
US-guided FNAB:
Sensitivity, 89.3% (p<.001)
Specificity, 82.9% (p<.001)
Accuracy, 86.9% (p<.001)
PPV, 87.2% (p<.001)
NPV, 6.1% (p<.001)
Inadequate biopsy rate, 6.1%
(p<.001)
Complications, None
Standard FNAB:
Sensitivity, 61.1% (p<.001)
Specificity, 73.3% (p<.001)
Accuracy, 65.0% (p<.001)
PPV, 100% (p<.001)
NPV, 61.1% (p<.001)
Inadequate biopsy rate, 19.6% (p<.001)
Complications, None |
Doyle
(case series) |
Ultrasound-guided LCNB with 14-gauge needle Stereotactic-guided LCNB with 14-gauge needle |
Stereotactic-guided LCNB:
110 nonpalpable lesions
2 palpable lesions
Ultrasound guided LCNB:
44 nonpalpable lesions
57 palpable lesions |
Absolute sensitivity 97%
Complete sensitivity 100% Absolute specificity 100%
Complete specificity 98.5% Positive predictive value 53% |
Liberman
(case series) |
Sonographic-guided CNB With 14-gauge automated Needle & a spring-loaded Automated gun, with long Excursion.
Stereotactic-guided CNB With 14-gauge automated Needle & a spring-loaded Automated gun, with long Excursion. |
Sonographic-guided CNB:
100 palpable lesions Stereotactic-guided CNB:
15 palpable lesions |
74% women spared additionaldiagnostic tissue sampling 97% agreement between malignant image-guided diagnoses & surgical excision 77% women with benign image-guided diagnoses were stable at follow-up. |
Pijnappel
(case series) |
Ultrasound-guided CNB With 14-gauge needle Using "long throw" biopsy Gun.
Stereotactic-guided CNB With 14-gauge needle Using "long throw" biopsy Gun. |
Ultrasound-guided CNB:
16 palpable lesions Stereotactic-guided CNB:
11 palpable lesions |
100% agreement between image-guided diagnoses of palpable lesions & histopathologic diagnoses of surgical specimen. |
Each of the clinical studies has flaws that limit the confidence that can be placed on their results. Sensitivity and specificity were not always specified, and not all methods of image-guidance or biopsy were evaluated. Failure to randomize subjects may have introduced biases in the studies reviewed. In some studies, statistical analyses were not provided.
The sensitivity and specificity of percutaneous breast biopsy without image guidance for breast lesions has been well-established, typically exceeding >85% in aggregate data for some methods. One would expect the use of image guidance to be at least as accurate as palpation guidance, because percutaneous biopsy is used in both instances. The question then becomes does image guidance improve diagnosis compared to palpation alone?
Even if image guidance improves diagnostic yield of biopsies of palpable breast lesions, does it lead to better patient management? For example, does it minimize the number of invasive procedures? Does it increase treatment options? Though the clinical studies did not directly address impact on patient management, greater diagnostic accuracy in this clinical circumstance is presumed to lead to a greater number of patients receiving appropriate treatment.
We can draw several conclusions from the evidence. First, one would presume that image-guided biopsy should be at least as accurate as palpation-guided biopsy for palpable lesions. However, the published studies do not support a conclusion that image-guided biopsy is more accurate than palpation-guided biopsy for all types of palpable breast lesions. For example, Hatada, et al. (1996) found no significant difference of diagnostic accuracy for lesions larger than 2 cm between ultrasound-guided FNAB and palpation-guided FNAB. While the other studies we reviewed assessed the accuracy of image-guided biopsy methods, they did not compare the accuracy of image-guided to palpation-guided biopsy using the same biopsy method, e.g., core needle biopsy. In the absence of a head-to-head comparison, firm conclusions about relative accuracy cannot be drawn. Second, although the published studies have some flaws, they suggest that image-guided biopsy is more accurate than palpation-guided biopsy for the histopathologic diagnosis of palpable lesions that are difficult to biopsy using palpation. In these cases, image guidance may reduce the number of false negative results, as compared to palpation guidance, due to the increased likelihood of proper placement of the biopsy device. Third, the specialty societies whose members use the technology uniformly support some role for image guidance for biopsying palpable lesions, although there is not consensus on the patient or lesion characteristics for which palpation alone is suboptimal. All suggest that it may minimize the number of false-negatives for some types of lesions. Unlike the other specialty societies, the ACS does not support coverage for all palpable lesions.
We believe the evidence is adequate to conclude that the use of percutaneous image-guided breast biopsy is clinically effective, and, therefore, reasonable and necessary, for patients with palpable lesions that are difficult to biopsy using palpation, but not for other types of palpable lesions. However, the available scientific evidence and expert opinion are not adequate to explicitly define what characteristics render a lesion difficult to biopsy using palpation. Therefore, the definition of "lesions that are difficult to biopsy using palpation" will be developed through the local policy mechanisms of the Medicare contractors. Many palpable lesions can be adequately biopsied using palpation alone. We would expect that only a limited number of patients with palpable lesions should require image-guided breast biopsy.
DECISION:
The Centers for Medicare and Medicaid Services has decided to issue a national coverage determination for the use of percutaneous image-guided breast biopsy of palpable lesions that are difficult to biopsy using palpation. Contractors have the discretion to decide what types of palpable lesions are difficult to biopsy using palpation.
1 Ultrasound is generally not an effective tool for routine breast cancer screening because sonographic imaging fails to detect microcalcifications. The main usefulness of ultrasound appears to be enhanced ability to distinguish between solid and cystic masses
2 "Percutaneous" means passage of something through the skin.
3 Most experts, however, estimate the error rate to be less than 5%.
4 A table which allows the patient to sit during the procedure also exists, although typically the patient lies prone.
5 There is a recognition that males also are diagnosed with breast cancer; for simplicity, patients are referred to as females in this document.
6 Pijnappel RM, Dalen A, Rinkes IH, et al. The diagnostic accuracy of core biopsy in palpable and non-palpable breast lesions. European Journal of Radiology 1997;24:120-123.
7 Hatada T, Aoki I, Okada K, et al. Usefulness of ultrasound-guided, fine-needle aspiration biopsy for palpable breast tumors. Archives of Surgery 1996;131:1095-1098.
8 Liberman L, Ernberg LA, Heerdt A, et al. Palpable breast masses: is there a role for percutaneous imaging-guided core biopsy? AJR 2000;75:779-787.
9 The patient was considered to have been spared additional diagnostic tissue sampling if percutaneous imaging yielded a benign finding for which surgery was neither recommended nor performed, or if percutaneous biopsy yielded carcinoma for which the patient underwent treatment without additional biopsy procedures.
10 Doyle AJ, King AR, Miller MV, and Collinc JP. Implementation of image-guided large-core needle biopsy of the breast on a limited budget. Australasian Radiology 1998;42:199-203.
11 Coding, however, is not part of the definition of a national coverage decision.
12 When assessing the evidence, we accord greater weight to clinical studies than to expert opinion, consistent with the principles of evidence-based medicine.
13 Specificity is used to rule in disease, whereas high sensitivity rules out disease.


