
CMS Electronic Prescribing for Controlled Substances (EPCS) Program
Measurement Year 2023 Compliance Status
For measurement year 2023, CMS sent informational notices to prescribers who met the program requirement only due to the declared national public health emergency. CMS sent the notices through email when possible and as a physical letter if there was no email address for the prescriber in the Provider Enrollment, Chain, and Ownership System (PECOS) or in the National Plan and Provider Enumeration System (NPPES).
CMS encourages prescribers to update their email and mailing addresses in PECOS and NPPES to ensure timely receipt of the notices and, therefore, the opportunity to submit a waiver application to become compliant. A prescriber’s non-compliance under the CMS EPCS Program may be considered in CMS processes for assessing potential fraud, waste, and abuse. Prescriber addresses are also used to determine who qualifies for the automatic declared disaster exception.
Prescribers who met the measurement year 2023 compliance only due to the declared national public health emergency had the option to submit a waiver request for additional extraordinary circumstances, such as technological limitations or other circumstances outside of their control.
The waiver application submission period was open from September 16, 2024, until Monday, November 18, 2024, at 8 p.m. ET.
For more on submitting a waiver application, see the CMS EPCS Program Waiver Application Fact Sheet.
CMS EPCS Policy in the Calendar Year 2025 Physician Fee Schedule Final Rule
CMS finalized the CMS EPCS Program policy that prescriptions written for a beneficiary in a long-term care (LTC) facility will not be included in determining compliance until January 1, 2028. CMS also finalized that compliance actions against prescribers who do not meet the compliance threshold based on prescriptions written for a beneficiary in an LTC facility will start measurement year 2028.
CMS EPCS Rules and Regulations
In October 2018, the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act (SUPPORT Act) was enacted into Public Law (115-271) to address the opioid crisis. Section 2003 of the SUPPORT Act generally mandates that Schedule II-V controlled substances under Medicare Part D and Medicare Advantage prescription drug (MA-PD) plans be prescribed electronically in accordance with an electronic prescription drug program. The CMS EPCS Program is separate from state EPCS program requirements.
Electronic prescribing for controlled substances enhances patient safety through patient identity checks, medication recommendations, and timely and accurate transmission of time sensitive prescriptions. EPCS also reduces prescriber burden by deterring and detecting prescription fraud and irregularities, improving workflow efficiencies, avoiding data errors, and reducing pharmacy calls to clarify written prescriptions.
Section 2003 of the SUPPORT Act provides the Secretary of the Department of Health and Human Services with discretion on whether to grant waivers or exceptions to the CMS EPCS requirement and gives the Secretary the authority through rulemaking to enforce and specify appropriate penalties for noncompliance with the CMS EPCS requirement. In November 2024, CMS released the Calendar Year 2025 Physician Fee Schedule final rule. For information about previous final rules, please see the EPCS Regulatory Milestones document in the “Downloads” section below.
CMS EPCS Program Timeline
The CMS EPCS Program timeline represents one measurement cycle, which is generally a 24-month period that consists of a measurement year, the compliance analysis period, and the notification period.
Figure 1. General Measurement Cycle
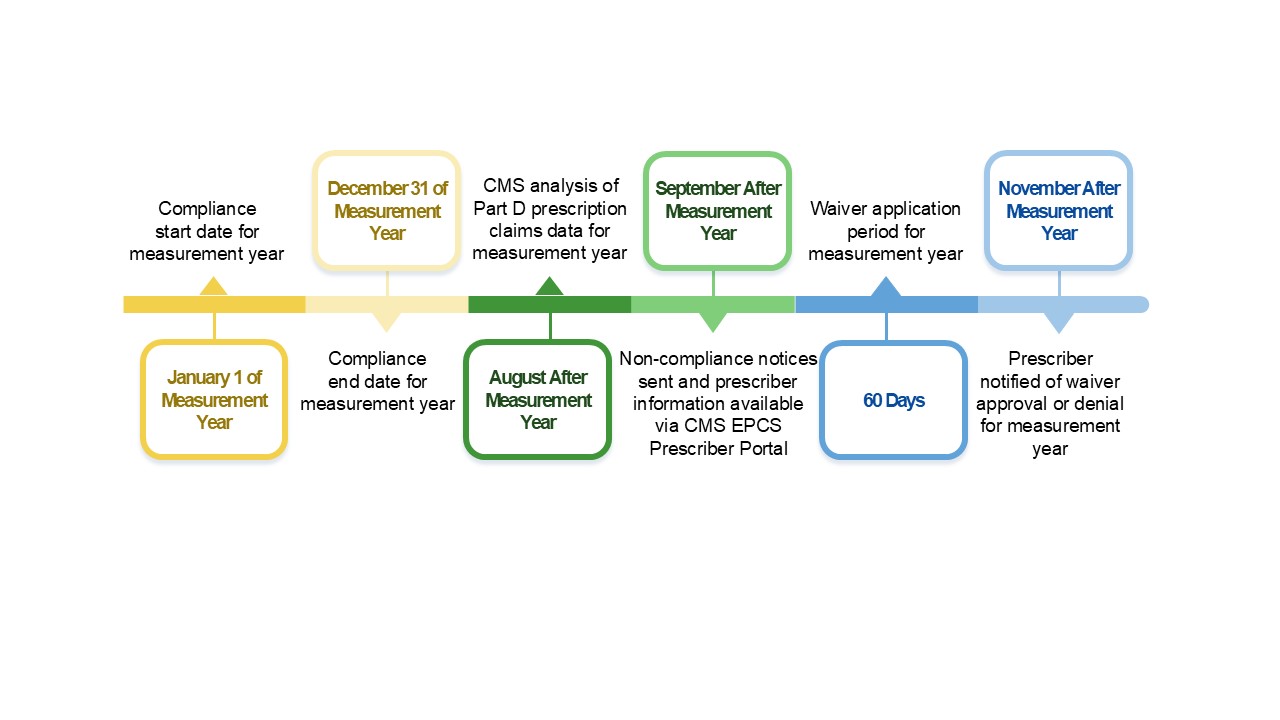
- November before measurement year – Physician Fee Schedule Final Rule released
- January 1 of measurement year – Compliance start date for measurement year
- December 31 of measurement year – Compliance end date for measurement year
- August after measurement year – CMS analysis of Part D prescription claims data for measurement year
- September after measurement year – Non-compliance notices sent and prescriber compliance status available via CMS EPCS Prescriber Portal
- September to November after measurement year – 60-day waiver application period for measurement year
- November after measurement year – Prescriber notified of waiver approval or denial for measurement year
Figure 2. Example: Measurement Year 2023 Cycle
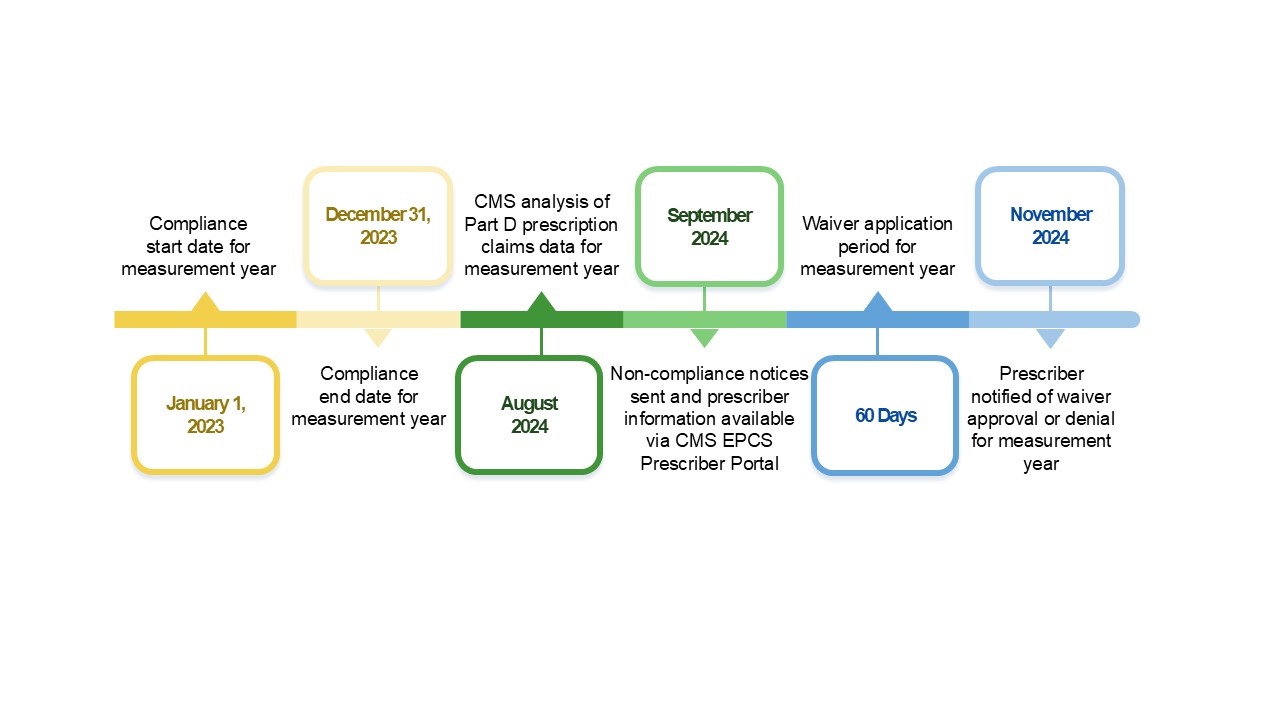
- November 18, 2022 – CY 2023 Physician Fee Schedule Final Rule released
- January 1, 2023 – Compliance start date for 2023 measurement year
- December 31, 2023 – Compliance end date for 2023 measurement year
- August 2024 – CMS analysis of Part D prescription claims data for 2023 measurement year
- September 2024 – Non-compliance notices sent and prescriber information available via an EPCS Prescriber Portal
- September to November 2024 – 60-day waiver application period for 2023 measurement year
- November 2024 – Prescriber notified of waiver approval or denial for 2023 measurement year
Compliance Overview
CMS EPCS Program Prescribers
Prescribers who issue prescriptions for Schedule II-V controlled substances to Medicare Part D beneficiaries from January 1 through December 31.
CMS EPCS Program Compliance Determination
CMS will analyze Medicare Part D Schedule II-V controlled substance prescription claims and use the prescriber’s National Provider Identifier (NPI) to measure compliance:

If the EPCS compliance rate is 70% or higher, the prescriber is considered compliant.
CMS EPCS Program Exceptions
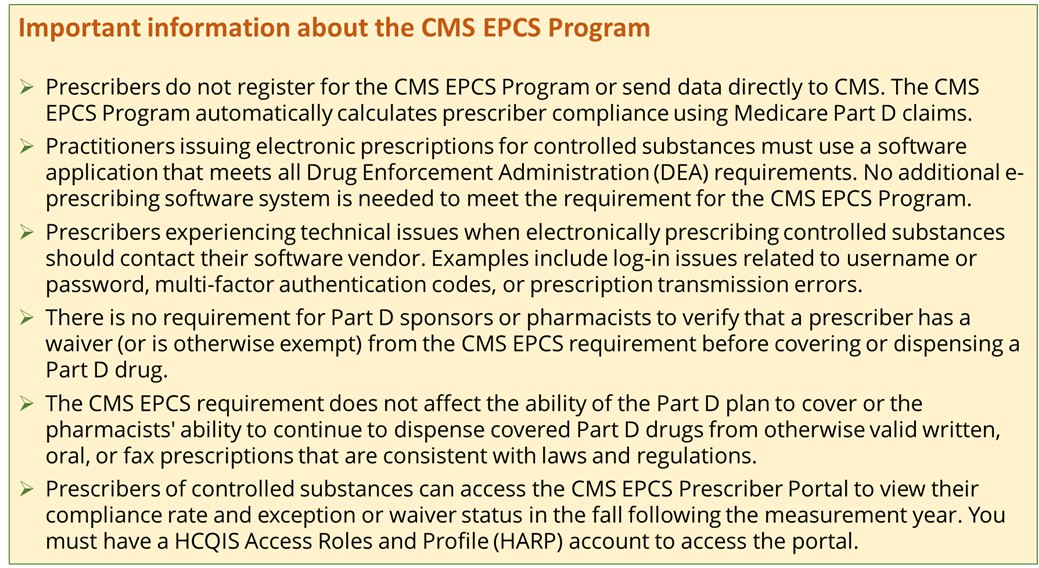
Small Prescriber Exception: CMS automatically provides this exception to prescribers who issue 100 or fewer qualifying Medicare Part D controlled substance prescriptions in the measurement year.
- Declared Disaster Exception: CMS automatically provides this exception to prescribers located in the geographic area of an emergency or disaster declared by a Federal, State, or local government entity. Starting in the 2024 measurement year, CMS will identify which emergencies or disasters qualify for this exception. CMS posts a list of the qualifying emergencies or disasters for each measurement year in the Downloads section below.
- CMS-Approved Waiver: CMS provides this exception to prescribers who submit and receive a CMS-approved waiver because the prescriber is unable to meet the CMS EPCS Program requirement due to circumstances beyond the prescriber's control.
Prescriptions written for a beneficiary in a long-term care (LTC) facility will be included in determining compliance no earlier than January 1, 2028.
Waiver
Prescribers or their representatives may request a measurement year waiver when circumstances beyond their control prevented them from electronically prescribing Schedule II-V controlled substances. Prescribers or their representatives may request a waiver in the CMS EPCS Prescriber Portal in the Fall after the measurement year (e.g., the 2023 measurement year waiver application period will open September 2024).
Non-Compliance Action
As the non-compliance action for each measurement year, CMS will send non-compliance notices to prescribers who have not met the CMS EPCS Program requirement. The notice will include information to prescribers that they are violating the CMS EPCS Program requirement, information about how they can come into a compliance, benefits of EPCS, and a link to the CMS EPCS Prescriber Portal where they can check compliance status and may request a waiver for circumstances beyond the prescriber’s control. A prescriber’s non-compliance under the CMS EPCS Program may be considered in CMS processes for assessing potential fraud, waste, and abuse, which, in some instances, could result in a referral to law enforcement or revocation of billing privileges, in the event that evidence of fraud, waste, or abuse is present. Notices will be sent by email, when possible, to available email addresses in PECOS and NPPES and by physical mail if there is no email address in PECOS or NPPES. CMS strongly recommends that all prescribers keep their email address accurate and up to date in both systems.

CMS EPCS Prescriber Portal
On the CMS EPCS Prescriber Portal, hosted on the Clinician Quality Reporting (CQR) website, a prescriber of Medicare Part D Schedule II–V controlled substances and their designated representatives can check the prescriber’s compliance status and submit a waiver application for the measurement year. Users can log in to the CMS EPCS Prescriber Portal with their HCQIS Access Roles and Profile (HARP) account user ID and password.
Once in the CMS EPCS Prescriber Portal, users can:
- Review the prescriber’s compliance status for the measurement year, including any automatic exceptions
- Submit a waiver application for the measurement year, if needed, based on circumstances beyond the prescriber’s control
Check the prescriber’s waiver application status
Although the prescriber’s compliance data for the measurement year won’t be available on the CMS EPCS Prescriber Portal until September of the following year (for example, 2023 measurement year data became available in September 2024), CMS suggests that users take one of the following actions:
- Reset their HARP account password. Registered users of the Quality Payment Program (QPP) should already have a HARP account. However, users may need to reset their HARP account password if they haven’t logged in to their account for 60 or more days.
- Create a HARP account. Users who don’t have a HARP account will need to create one to log in to the EPCS Prescriber Portal.
For more information, review the CMS EPCS Prescriber Portal & Waiver Application User Guide.
To stay up to date on the CMS EPCS Prescriber Portal, subscribe to the CMS EPCS Program Listserv.
See “Downloads and Related Links” for more resources.
CMS encourages prescribers to subscribe to the EPCS listserv to stay up to date on the CMS EPCS Program. Click here to subscribe.
See "Downloads and Related Links" for more resources.
Where Can I Get Help? To contact the CMS EPCS Service Center:
|
Downloads
-
EPCS Program Getting Started Quick Reference Guide (PDF) -
EPCS Frequently Asked Questions (PDF) -
EPCS Glossary (PDF) -
EPCS Regulatory Milestones (PDF) -
CMS EPCS Program Guidance Regarding the Role of the Pharmacy/Pharmacist (PDF) -
Waiver Application Quick Reference Guide (PDF) -
2023 EPCS Declared Disasters Fact Sheet (PDF) -
2023 Measurement Year CMS EPCS Program Requirements At-A-Glance (PDF) -
2024 Measurement Year CMS EPCS Program Requirements At-A-Glance (PDF) -
2024-01-11 CMS EPCS Program Updates and CY2024 PFS Final Rule Webinar Slides (PDF) -
2024-01-11 CMS EPCS Program Updates and CY2024 PFS Final Rule Webinar Transcript (PDF) -
2024-01-11 CMS EPCS Program Webinar Q&A (PDF) -
2023-01-12 CMS EPCS Webinar Q & A (PDF) -
2023-01-12 CMS EPCS Webinar Transcript (PDF) -
2023-01-12 Introduction to the CMS EPCS Program Slides (PDF) -
2023-07-27 CMS EPCS Proposed Rule Webinar Transcript (PDF) -
2023-07-27 CMS EPCS Program Webinar Slides - Handout (PDF)